# 2799
This is third and last part of my extended look at the recent PNAS paper entitled Early and sustained innate immune response defines pathology and death in nonhuman primates infected by highly pathogenic influenza virus by Carole Baskin et. al., which analyses the pathogenesis of the H5N1 virus compared to seasonal flu, and 1918-like viruses.
- In Part I, which appeared on Saturday, we examined the 4 different viruses used in this study, and the methods used by the research team.
- In Part II, which appeared on Sunday, we looked at the innate immune system and began looking at the results of this study.
This series is not intended to be a scientific review, but instead a layman's tour of the paper.
Today, we'll finish looking at the findings, and talk about about what it might mean. In Part II I listed the 5 major findings, and we discussed the first 3.
Today we'll cover points 4 & 5.
4. The H5N1 infection also resulted in prolonged margination of circulating T lymphocytes and notable apoptosis of activated dendritic cells in the lungs and draining lymph nodes early during infection.
The loss of dendritic cells (named after, but not to be confused with the projections from neurons) through premature apoptosis (programmed cell death) in the lungs and draining lymph nodes is viewed by these researchers as a key finding.
Dendritic cells are a special type of immune cell, most commonly found in tissues that are exposed to the outside environment (skin, lungs, digestive tract), that boosts immune responses by showing antigens on its surface to other cells of the immune system.
In the simplest language, dendritic cells help `teach' the adaptive immune system how to recognize a virus, and are key in the creation of pathogen-specific antibodies.
A loss of dendritic cells at the site of infection (lungs) could negatively impact the body's ability to generate post-infection immunity.
In other words, catching and surviving the virus may not guarantee future immunity to the virus.
5. While both 1918 reassortant viruses also were highly pathogenic, the H5N1 virus was exceptional for the extent of tissue damage, cytokinemia, and interference with immune regulatory mechanisms, which may help explain the extreme virulence of HPAI viruses in humans.
The primates infected with the H5N1 virus began to experience rapid, serious, and potentially permanent tissue damage in the lungs.
Unlike the seasonal, and 1918-like viruses which attacked type I pneumocytes, the H5N1 virus seems to target type II pneumocytes.
Type II pneumocytes are not only more numerous in the lungs, they are responsible for producing surfactant with antimicrobial, immunomodulatory, and anti-inflammatory properties.
A loss of type II pneumocytes severely degrades the lungs ability to fight off an infection, and to repair damaged tissue.
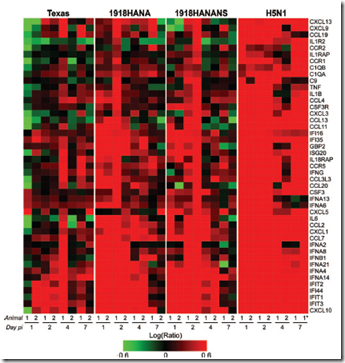
The most widely published graphic from this study has been this dramatic representation of the relative generation of 45 key cytokines in the host animals.
Red, as you might imagine, indicates a greater production of cytokines. Green signals a reduction.
Seasonal influenza (H1N1-Texas) and the two 1918-like ressortants all generated increased cytokine production - particularly in the first 4 days of infection.
By day 7, however, the production of cytokines was fading.
In terms of quantity, the 1918-like viruses produced considerably higher numbers of cytokines than the seasonal virus.
But the expression of cytokines by hosts infected with the H5N1 virus elevates very early in the infection, and remains elevated across the board for the entire 7 day observation period.
Both in terms of quantity, and duration, the H5N1 virus elicits an extreme immune response in the host animals.
The authors refer to this it as early, sustained, and relentless.
Which pretty well describes it.
The authors of this study summarize these findings this way:
In conclusion, H5N1 virulence is a multipronged mechanism that causes severe lung pathology with potentially permanent tissue damage within 24 h PI, accompanied by excessive and sustained type I IFN, inflammation, and innate immune induction.
* * * * * * *
In November of 2006 I wrote a blog called Not Your Father’s Influenza , which outlined in very broad terms, some of the differences between seasonal influenza and the (thus far) highly lethal H5N1 virus.
After reading WHO (World Health Organization) report entitled Influenza Research At the Human And Animal Interface, I wrote:
While H5N1 is indeed an influenza virus, calling it the `flu’ is like calling a Hurricane a patch of bad weather.
It doesn’t even begin to describe it.
Obviously, with a CFR (case fatality ratio) of 60% among identified human infections, we've known for some time that the H5N1 virus isn't any ordinary flu.
Today, courtesy of this research by Carole Baskin et. al., we now have a much better understanding of Why the virus is so deadly.
And it turns out, there isn't just one thing, but rather a host of reasons.
From a scientific standpoint, all of this is fascinating. We are not only seeing a unique pathogenesis, we are learning more and more about how the immune system works.
But the question I'm sure everyone is asking is:
If the H5N1 virus ever goes pandemic, will it retain this unprecedented lethality?
We know that the H5N1 virus must undergo some (unknown) number of genetic changes in order to become easily transmissible. The hope is, these changes will also reduce its virulence.
It is a popularly held theory that an outbreak from a virus that is too deadly will burn itself out quickly. To spread effectively, you need mobile, yet infectious, vectors.
A disease that sickens, incapacitates, and kills too quickly loses too many opportunities to spread. Many researchers point to the localized, but short-lived outbreaks of Ebola in central Africa as prime examples.
But not everyone is convinced that this virulence for transmissibility tradeoff is inevitable.
There are simply no guarantees.
Of course, there are no guarantees that the H5N1 virus will ever become a pandemic strain.
The next pandemic could well come from an H2, or an H7, or even an H9 virus, or perhaps from a reassortment of the H5 virus with another strain.
But whatever the cause, this research has provided us with a much more detailed look at the pathogenesis of influenza viruses in non-human primates.
And that will hopefully help doctors work on new avenues of treatment.