BSL-3 – Credit CDC PHIL
# 6979
Seven years ago, when the H5N1 virus suddenly leapt out of a handful of southeast Asian countries to invade parts of Europe and the Middle East, pandemic concerns were understandably heightened.
As a result many countries hastened to create - or acquire - an emergency stockpile of experimental bird flu vaccine.
In 2007, I wrote:
Switzerland has reportedly purchased 8 million doses of pre-pandemic vaccine, enough to inoculate their entire citizenry. Denmark, it was widely reported last January, ordered in enough pre-pandemic vaccine for half of their population. And in Australia, there has been talk of inoculating their entire nation.
New Zealand recently announced they had enough pre-pandemic vaccine on hand for 100,000 essential workers, and the UK is exploring the purchase of large quantities of vaccine.
While the WHO has never endorsed the idea, obviously there are a good many nations that view having a pre-pandemic vaccine as being a matter of national security.
Japan, Taiwan, the United States and others eventually acquired small to medium-sized quantities of H5N1 vaccine over the next couple of years.
With only a limited shelf life (usually around 3 years) - and based on an older clade of the virus - those early stockpiles have either already expired or will shortly.
While the feared bird flu pandemic has thus far failed to materialize, some countries have adopted a `use it or lose it’ strategy, offering the aging H5N1 jab to health care workers, veterinarians, and other high risk groups (see Hong Kong: H5N1 Vaccine Recommended For Certain Lab Workers).
Countries opting to `store’ their vaccine stockpile in the arms of healthcare and public safety workers have included Japan (see Japan Begins Pre-Pandemic Inoculation Of Health Care Workers) and Taiwan (see Taiwan Offers Public Bird Flu Vaccinations.)
Over the weekend, after the spate of H5N1 cases reported in Cambodia since the New Year, Taiwan once again announced plans to provide their H5N1 vaccine to certain high risk individuals.
Certain groups entitled to free avian flu vaccinations
2013/03/02 15:24:22Taipei, March 2 (CNA) Taiwan has begun to provide free vaccinations against avian influenza H5N1 to people at high risk of contracting the deadly virus, such as laboratory staff, a senior health official said Saturday.
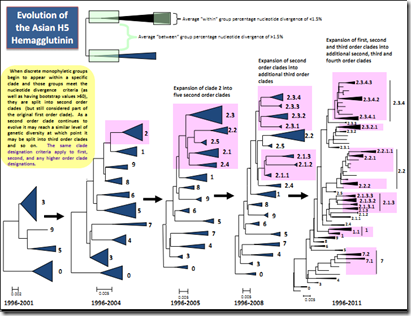
Just as with the seasonal flu, the H5N1 virus evolves into new clades, meaning that updated vaccines must be created and tested. The following chart from the World Health Organization shows just how much diversity the virus has acquired.
While not garnering the sort of headlines that we saw in 2006-2007, researchers continue to work on creating and testing the next generation of bird flu vaccines.
Which brings us to a press release, sent to me by a reader, regarding a recent call (January, 2013) for volunteers to take part in an H5N1 vaccine clinical trial being conducted by the Wesley Research Institute in Queensland, Australia.
Wesley Research Institute conducts new bird flu vaccine study
Posted on January 14, 2013
The Wesley Research Institute Clinical Trials Centre is seeking volunteers aged 65 and over to participate in a new avian influenza (bird flu) vaccine study that may prevent the spread of infection and death.
H5N1 type influenza virus is a specific strain that resulted in rapid spread of influenza in birds and poultry (bird flu) across Asia, Europe and Africa. This strain has been associated with over 550 human cases (spread from animals) and about 320 deaths.
The number of Australians travelling overseas has grown at an unprecedented rate over recent years. 70% of Australians 60 years old and over, taking a holiday overseas in the last 12 months visited or at least stopped over at the Pacific Asia region where in 1997 the first outbreak of bird flu occurred.
The symptoms of bird flu are similar to those of other types of flu and the onset is sudden. The time from infection to the start of symptoms is usually three to five days, although in some cases it can be up to seven days. Symptoms usually last for up to a week. In many cases, bird flu can cause rapid deterioration, pneumonia (inflammation of the tissue of one or both lungs) and multiple organ failure, all of which can be fatal.
Travel safe, keep bird flu away!
You may be suitable to participate if:
- Generally healthy
- Aged 65 years old and over
- Able to attend visits at The Wesley Hospital in Auchenflower
Study volunteers will be compensated for their time and travel.
While the H5N1 virus remains a major concern, there are other avian and swine influenza viruses that are being watched closely for signs of pandemic potential as well. Accordingly, the World Health Organization regularly updates their list of candidate flu strains for vaccine research and development.
Antigenic and genetic characteristics of A(H5N1), A(H7N3), A(H9N2) and variant influenza viruses and candidate vaccine viruses developed for potential use in human vaccines
February 2013
This summary provides a review on the A(H5N1), A(H7N3), A(H9N2) and variant influenza virus activity and virus characterization, and describes the current status of the development of candidate vaccine viruses for pandemic preparedness purposes. It is meant to provide guidance for national authorities and vaccine companies on the selection of candidate viruses for use in vaccine development.