# 2217
For several years I’ve urged my readers to talk to their doctors about whether getting the 23-valent pneumococcal (Pneumovax) vaccine is appropriate for them.
Seven Steps You Can Take Now To Prepare For A Pandemic
It Doesn't Have To Be Pandemic Flu
With Flu Season Upon Us
It isn’t universally recommended by the CDC, but for many people in a variety of risk groups, it can be an important preventative against many causes of secondary bacterial pneumonias.
Even if you aren’t sure if you fall into one of the risk groups, you may wish to discuss this option with your family physician.
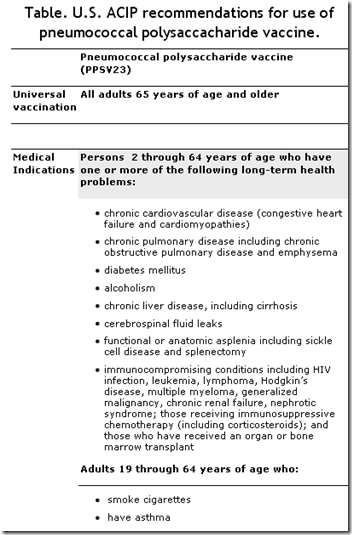
Yesterday, the CDC updated their recommendations in light of the current outbreak of H1N1. I’ve excerpted portions of those recommendations below.
Interim guidance for use of 23-valent pneumococcal polysaccharide vaccine during novel influenza A (H1N1) outbreak
June 9, 2009 2:15 PM ET
Objective
To provide interim guidance on which groups should be vaccinated with the 23-valent pneumococcal polysaccharide vaccine (PPSV23) to prevent pneumococcal infections during the outbreak of novel influenza A(H1N1).
Background
Influenza predisposes individuals to bacterial community-acquired pneumonia. During the 20th century influenza pandemics, secondary bacterial pneumonia was an important cause of illness and death and Streptococcus pneumoniae (pneumococcus) was reported as the most common etiology. Severe pneumococcal pneumonia associated with inter-pandemic influenza also has been reported, and S. pneumoniae remains a leading cause of vaccine-preventable illness and death in the United States.
<SNIP>
Pneumococcal vaccines
During influenza outbreaks, pneumococcal vaccines may be useful in preventing secondary pneumococcal infections and reducing illness and death. Currently, two vaccines are available for prevention of pneumococcal disease, a 23-valent pneumococcal polysaccharide vaccine (PPSV23) and a 7-valent pneumococcal conjugate vaccine (PCV7).
<SNIP>
Pneumococcal conjugate vaccines
PCV7 is recommended for all children aged less than 5 years; national coverage among 19-35 month olds with 3 or more PCV7 doses is currently > 90% (National Immunization Survey, July 2007-June 2008). PCV7 coverage estimates are available at: http://www.cdc.gov/vaccines/stats-surv/nis/data/tables_0708.htm .
While maintaining this high coverage is important, expanding the use of PCV7 to people aged ≥ 5 years is not indicated because circulation of the 7 serotypes included in the vaccine has declined substantially and disease caused by these serotypes is now uncommon.
For further information about recommendations for use of pneumococcal vaccines, including contraindications, precautions and adverse effects please see the following:
- Recommended adult immunization schedule - United States, 2009
- Pneumococcal polysaccharide vaccine. Vaccine information statement (VIS)
- Centers for Disease Control and Prevention. Prevention of pneumococcal disease. MMWR Morbidity and Mortality Weekly Report 1997;46(RR-8):1-20.
- ACIP provisional recommendations for use of pneumococcal vaccines.