# 5924
I hadn’t intended today to be a `theme’ day at AFD, but things have certainly turned out that way.
I started early this morning with a study on the reduced immune response from the flu vaccine among the obese (here), and this afternoon posted a link to the on-target comments by Dr. Jody Lanard on the CDC’s recent rollout of vaccine effectiveness numbers (here).
For the hat trick I’ve saved the best for last.
A comprehensive study lead by Michael T. Osterholm, director of CIDRAP (the Center for Infectious Disease Research and Policy) at the University of Minnesota, that provides a systematic review and meta-analysis on the efficacy and effectiveness of the TIV (trivalent Inactivated Vaccines) and LAIV (Live attenuated influenza vaccine) influenza vaccines.
Note: Most of the time, the terms `effectiveness’ and `efficacy’ can be used interchangeably, but in the scientific world there are subtle differences.
Effectiveness describes how well something works under day-to-day, real-world conditions. In the following study, it is used to gauge the results of observational studies.
Efficacy describes how well something performs in a more controlled setting – and here it is used to describe the results of randomized placebo-controlled clinical trials.
Researchers at CIDRAP, along with colleagues from the Marshfield Clinic Research Foundation and Johns Hopkins University examined more than 5700 existing vaccine studies going back to 1967, and found 31 that met their (admittedly strict) criteria for inclusion.
What they were looking for were well-mounted studies that used highly sensitive laboratory testing (RT-PCR or culture) to confirm influenza infection in its participants.
Since the flu vaccine cannot be reasonably be expected to protect against non-influenza respiratory illness (ie. rhinovirus, adenovirus, enteroviruses, etc.), studies that fail to do this sort testing are of considerably less value.
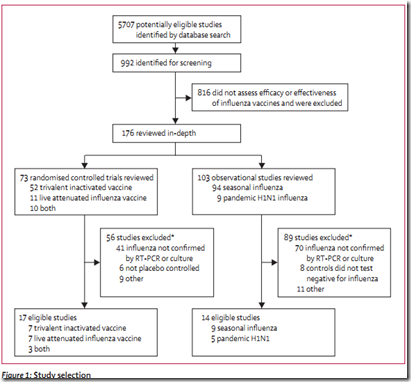
After an exhaustive examination and whittling down process, fewer than 3 dozen studies met their criteria. As a result there remain some significant gaps in the evidence.
For example, there were no randomized controlled trials (RTCs) showing efficacy of the TIV (trivalent Inactivated Vaccines) in those aged 2–17 years or in adults 65 years or older.
Similarly, they found no RCTs showing the efficacy of the LAIV (Live attenuated influenza vaccine) for people aged 8–59 years.
In the discussion, the authors write:
The evidence from trials and observational studies suggests that presently available influenza vaccines can provide moderate overall protection against infection and illness, with LAIV providing a consistently higher level of protection in children aged 7 years or younger.
But they found the protective effects of the flu vaccine could vary considerably from one season to the next, and among different age groups. Some years, and in some cohorts, there was little evidence of protection.
Although some of the numbers are lower than have been commonly stated in the past, today’s flu vaccines were shown to provide a moderate level of protection.
TIV showed efficacy in preventing influenza during 8 of 12 flu seasons (67%) with a combined efficacy of 59% among healthy adults (aged 18–65 years).
And among children aged 2-7, the LAIV proved even more protective, showing efficacy in 9 out of 12 flu seasons (75%) with a pooled efficacy of 83%.
Beyond the numbers, which probably represent the most accurate assessment of influenza vaccine effectiveness to date, this study also highlights the folly of depending upon a vaccine technology that has changed little since the 1950s.
Not only does it take too long to produce an emergency vaccine in the face of a pandemic . . . those at greatest risk from a novel influenza virus are likely to receive the least amount of benefit from today’s vaccines.
The study’s authors say their findings should be seen as a clarion call for the development of more effective influenza vaccines.
Until that can happen, and despite these lower efficacy numbers, they still recommend getting the seasonal flu vaccine.
Robert Roos, News Editor for CIDRAP (which operates independently of CIDRAP’s research and policy programs) provides a good deal more detail on all of this, including extensive remarks by Dr. Osterholm.
Strict meta-analysis raises questions about flu vaccine efficacy
Robert Roos News Editor
Oct 25, 2011 (CIDRAP News) – A rigorous new analysis of 44 years' worth of studies is raising questions about the evidence for the effectiveness of influenza vaccines in elderly people and, for certain types of vaccines, in children and younger adults as well.
Applying very strict criteria to filter out potential bias and confounding, a US research team sifted more than 5,000 studies and found only 31 that they felt provided reliable evidence about the efficacy and effectiveness of flu vaccines. The findings were published online today by Lancet Infectious Diseases.
(Cites)
Osterholm MT, Kelley NS, Sommer A, et al. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2011 (published online Oct 25)
Kelly H, Valenciano M. Estimating the effect of influenza vaccines. (Commentary) Lancet Infect Dis 2011 (published online Oct 25)