# 6631
Yesterday evening the CDC published an Early Release MMWR report on the steroid-related outbreak of fungal meningitis across the country. We are provided with details on the ongoing investigation, a breakdown of symptoms and onset dates for a number of cases, and demographic data on many of the patients.
It’s a lengthy report, and so I’ve only excerpted a small portion (reparagraphed for readability). Follow the link to read it in its entirety.
Multistate Outbreak of Fungal Infection Associated with Injection of Methylprednisolone Acetate Solution from a Single Compounding Pharmacy — United States, 2012
Early Release
October 12, 2012 / 61(Early Release);1-4On September 18, 2012, the Tennessee Department of Health was alerted by a clinician regarding a patient with culture-confirmed Aspergillus fumigatus meningitis diagnosed 46 days after epidural steroid injection at a Tennessee ambulatory surgical center.
By September 27, the initial investigation, carried out by the Tennessee Department of Health in collaboration with CDC and the North Carolina Department of Health and Human Services, had identified an additional eight patients with clinically diagnosed, culture-negative meningitis: seven in Tennessee and one in North Carolina.
All nine patients had received epidural steroid injection with preservative-free methylprednisolone acetate solution (MPA), compounded at New England Compounding Center (NECC) in Framingham, Massachusetts. All nine patients had received one or more injections from three lots of MPA (lot numbers 05212012@68; 06292012@26; and 08102012@51). As of October 10, a multistate investigation led by CDC in collaboration with state and local health departments and the Food and Drug Administration (FDA) had identified 137 cases and 12 deaths associated with this outbreak in 10 states.
Active case-finding efforts and extensive investigation into medications and medication lot numbers received by patients have confirmed that, as of October 10, no cases were associated with other lots of MPA, nor were any associated with other NECC products. This report describes the ongoing investigation by CDC and state and local health departments, and includes important recommendations for physicians and patients.
Onset of Symptoms of 61 cases. Earliest August 18th, 2012.
Of the 70 cases where information is known, the median age of those affected is 68 (range 23-91). Women outnumber men by more than 2 to 1 (M=22, F=48), and the median incubation period between getting the injection and developing symptoms is 15 days (max=42).
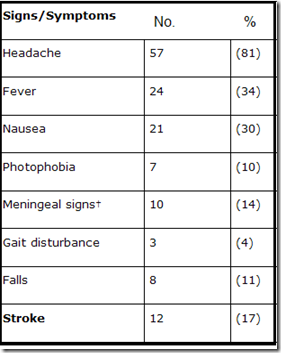
Presenting signs and symptoms (n=70)
Again, from the report:
As of October 10, evidence of a fungal infection had been found in 26 (37%) patients by culture, histopathology, or polymerase chain reaction.
The fungal species had been identified in 14 patients; Exserohilum spp was identified in 13, and Aspergillus fumigatus was identified in one patient (Table).
As I mentioned yesterday, Exserohilum has never been associated with meningitis before, and so doctors are still trying to determine the optimum treatment.
Exserohilum rostratum – Credit CDC
The drugs used to combat these fungal infections (voriconazole and lipsomal amphotericin B.) are very strong, and not without the risk of adverse effects.
Doctors are warning that the treatment regimen for these types of infections may be prolonged, and difficult.
And finally, the editorial comment portion of this MMWR report cautions:
Although available preliminary data demonstrate incubation periods ranging from 4 to 42 days, the maximum incubation period for this infection is not known; therefore, asymptomatic but exposed patients should remain vigilant for symptoms and seek medical attention should symptoms develop.
More guidance for patients and clinicians, including interim treatment guidelines, is available at http://www.cdc.gov/hai/outbreaks/meningitis.html.