Photo Credit FDA
# 6796
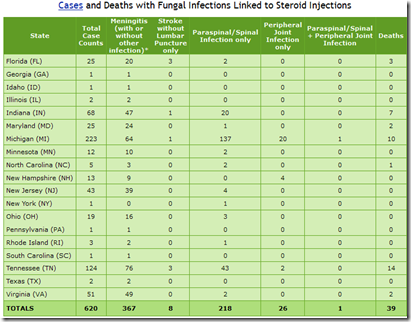
While the number of new meningitis cases linked to contaminated steroid injectables produced by NECC have slowed in recent weeks after their recall in late September, the number of slower-to-develop paraspinal/spinal & peripheral joint (site of injections) infections continues to rise.
In many cases, the symptoms of fungal infection are subtle - and since these patients (by definition) already had joint/spinal pain when they received these injections - it isn’t always easy to identify patients who develop pockets of these slow growing organisms.
The CDC, in reviewing diagnostic imaging of a number of patients who received these injections, has determined that there may be a substantial number of as-yet unrecognized infections out there.
So today they have issued a HAN Health Update.
December 20, 2012, 11:55 ET (11:55 AM ET)
CDC HAN-0338-2012-12-20-U-NUpdate: Multistate Outbreak of Fungal Infections among Persons Who Received Injections with Contaminated Medication
Summary
New information from diagnostic imaging of patients exposed to contaminated methylprednisolone acetate (MPA1) from the New England Compounding Center (NECC) in Framingham, Mass., demonstrates the need for assertive clinical evaluation of these patients for the possibility of an unrecognized, localized spinal or paraspinal infection. This Health Alert Network (HAN) notice provides updated guidance and information about the ongoing multistate outbreak of fungal infections as follows:
- CDC and state partners have analyzed new preliminary data based on recent Magnetic Resonance Imaging (MRI) studies among patients who had spinal or paraspinal injection with contaminated MPA from NECC. These findings demonstrate that among patients with no previous evidence of infection, and with new or worsening symptoms at or near the site of their injection, more than 50% had findings suggestive of a localized spinal or paraspinal infection, including epidural abscess, phlegmon, arachnoiditis, discitis, or vertebral osteomyelitis.
- This new information suggests that some patients who received spinal or paraspinal injections with implicated MPA from NECC may currently have an unrecognized, localized spinal or paraspinal infection.
- CDC is therefore re-emphasizing the guidance from the November 20 HAN advisory that recommended clinicians remain vigilant for evidence of fungal infection in these patients and use an assertive approach for clinical management and follow-up of these patients. CDC continues to recommend MRI with contrast of the symptomatic area(s) in patients with new or worsening symptoms at or near their injection site following spinal or paraspinal injection of implicated MPA.
- In addition, CDC is recommending that clinicians should consider obtaining an MRI with contrast of the injection site in patients with persistent but baseline symptoms because the presentation of these spinal or paraspinal infections can be subtle and difficult to distinguish from a patient’s baseline chronic pain.
The CDC’s Health Alert Network (HAN) is designed to ensure that communities, agencies, health care professionals, and the general public are able to receive timely information on important public health issues.
You can sign up for HAN messages, and scores of other CDC and HHS email notifications, by going to the CDC - Quick Subscribe GovDelivery page.
There are 4 types of HAN releases, starting from the highest priority to the lowest.
- Health Alert - Conveys the highest level of importance; warrants immediate action or attention.
- Health Advisory - Provides important information for a specific incident or situation; may not require immediate action.
- Health Update - Provides updated information regarding an incident or situation; unlikely to require immediate action.
- Info Service -Provides general information that is not necessarily considered to be of an emergent nature.
Complicating matters, last week the FDA updated their list of contaminates detected in injectable drugs produced by this compounding pharmacy.
Laboratory Testing and Results
[12-12-2012] FDA and CDC have identified bacterial and/or fungal contamination in unopened vials of betamethasone, cardioplegia, and triamcinolone solutions distributed and recalled from NECC. These include bacteria known as Bacillus, and fungal species including Aspergillus tubingensis, Aspergillus fumigatus, Cladosporium species,and Penicillium species.
Although rare, some of the identified Bacillus species can be human pathogens. Some of the fungal organisms identified, particularly Aspergillus fumigatus, are known to cause disease in humans. It is not known how product contamination with these organisms could affect patients clinically.