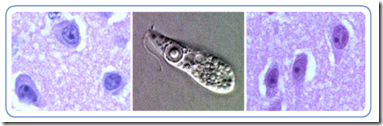
L & R: Trophozoites of N. fowleri in brain tissue, stained with H&E. Center: Ameboflagellate trophozoite of N. fowleri. Credit: DPDx
#7596
Amoebic infections by Naegleria fowleri resulting in PAM (Primary Amebic Meningoencephalitis) or by Acanthamoeba and Balamuthia mandrillaris producing GAE (Granulomatous amoebic encephalitis) are fortunately exceedingly rare, but for the unlucky few who are infected, survival is rare.
The CDC only reports one survivor of Naegleria fowleri out of 128 cases diagnosed in the United States (cite) over the past 50 years.
Survival from GAE is very rare as well, and those few who do survive usually experience significant neurological impairment.
This summer, we’ve already seen a couple of Naegleria infections linked to swimming in freshwater lakes reported in the United States (see Florida Reports Naegleria fowleri Infection, Naegleria fowleri Shuts Water Park), and last year, we saw two cases in Louisiana linked to nasal irrigation using tap water (see FDA Advice On Safe Use Of Neti Pots).
Until recently, treatment options have been limited to supportive care, but now the CDC is making available a drug (Miltefosine)– currently used outside of the U.S. to treat visceral and cutaneous leishmaniasis – to victims of FLA (Free Living Amoebic) in the United States.
While there’s not a lot of history of using this drug to treat these infections, the CDC believes there is enough evidence that it provides a `survival advantage’ to have imported the drug for this use.
Here is the CDC MMWR release from yesterday:
Investigational Drug Available Directly from CDC for the Treatment of Infections with Free-Living Amebae
Weekly
August 23, 2013 / 62(33);666-666Infections caused by free-living amebae (FLA) are severe and life-threatening. These infections include primary amebic meningoencephalitis (PAM) caused by Naegleria fowleri* and granulomatous amebic encephalitis caused by Balamuthia mandrillaris† and Acanthamoeba species.§ Although several drugs have in vitro activity against FLA, mortality from these infections remains >90% despite treatment with combinations of drugs.
Miltefosine is a drug used to treat leishmaniasis and also has shown in vitro activity against FLA (1), but as an investigational drug, it has not been readily available in the United States. With CDC assistance, however, miltefosine has been administered since 2009 for FLA infections as single-patient emergency use with permission from the Food and Drug Administration. Although the number of B. mandrillaris and Acanthamoeba species infections treated with a miltefosine-containing regimen is small, it appears that a miltefosine-containing treatment regimen does offer a survival advantage for patients with these often fatal infections (2). Miltefosine has not been used successfully to treat a Naegleria infection, but the length of time it has taken to import miltefosine from abroad has made timely treatment of fulminant Naegleria infections difficult.
CDC now has an expanded access investigational new drug (IND) protocol in effect with the Food and Drug Administration to make miltefosine available directly from CDC for treatment of FLA in the United States. The expanded access IND use of miltefosine for treatment of FLA is partly supported by 26 case reports of FLA infection in which miltefosine was part of the treatment regimen (Division of Foodborne, Waterborne, and Environmental Diseases, National Center for Emerging and Zoonotic Infectious Diseases, CDC, unpublished data, 2013). Miltefosine generally is well-tolerated, with gastrointestinal symptoms the most commonly reported adverse effects. Clinicians who suspect they have a patient with FLA infection who could benefit from treatment with miltefosine should contact CDC to consult with an FLA expert. For diagnostic assistance, specimen collection guidance, specimen shipping instructions, and treatment recommendations, clinicians should contact the CDC Emergency Operations Center at 770-488-7100.
Reported by
Corresponding contributor: Jennifer R. Cope, Div of Foodborne, Waterborne, and Environmental Diseases, National Center for Emerging and Zoonotic Infectious Diseases, CDC, jcope@cdc.gov, 404-718-4878.