# 10,991
The World Health Organization - in conjunction with their bi-weekly global influenza update - has released a special risk assessment on the predominant flu virus across much of the globe this year; A(H1N1)pdm09.
Over the past several weeks we've been monitoring the emergence of a new subgroup of clade 6B of H1N1pdm09 in Russia, raising questions over its antigenic match to the current vaccine.
According to the WHO, while they acknowledge that new sub-groups of A(H1N1) have emerged, the `majority of the 6B viruses' tested still remain antigenically similar to the vaccine virus.
So far, phylogenetic analysis of the haemagglutinin (HA) demonstrated that the HA genes of all viruses collected since September 2015 belong to genetic subgroup 6B. Within 6B, sub-subgroups with shared amino acid changes have emerged. Despite the genetic evolution of A(H1N1)pdm09 viruses, the majority of 6B viruses including those in the emerging sub-subgroups, remain antigenically1 closely related to the vaccine virus
The full statement follows:
Risk Assessment - Seasonal Influenza A(H1N1)pdm09
8 February 2016
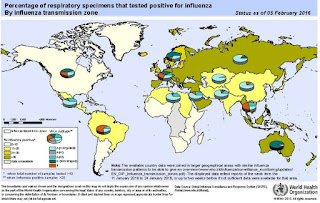
Compared to previous years, northern hemisphere seasonal influenza activity commenced late in some countries in western Europe, North America and eastern Asia. Transmission, as demonstrated by influenza-like illness (ILI) rates, has started to exceed country-specific baseline rates, but is still relatively low in general with the exception of some eastern European countries where a sharp increase of ILI rates has been observed and countries in western Asia where influenza activity may have already peaked.
Among the currently circulating seasonal influenza viruses in the temperate zone, influenza A(H1N1)pdm09 virus is predominating, except in northern China where influenza A(H3N2) and influenza B viruses are widely co-circulating though the proportion of A(H1N1)pdm09 virus is increasing. In a few European countries, influenza A(H3N2) and influenza B viruses are also circulating.
In some countries there have been reports of hospitalizations with severe disease associated with influenza A(H1N1)pdm09 virus infections. Based on the WHO global influenza surveillance, in countries with influenza A(H1N1)pdm09 virus predominating, the hospitalization and intensive care unit (ICU) admission patterns seem to be similar to previous seasons when this virus predominated and where young/middle-aged adults experienced severe disease.
The WHO Collaborating Centres for Influenza (CCs) of the WHO Global Influenza Surveillance and Response System (GISRS) have characterized influenza A(H1N1)pdm09 viruses collected from more than 30 countries since September 2015, including those from countries reporting severe infections. So far, phylogenetic analysis of the haemagglutinin (HA) demonstrated that the HA genes of all viruses collected since September 2015 belong to genetic subgroup 6B. Within 6B, sub-subgroups with shared amino acid changes have emerged. Despite the genetic evolution of A(H1N1)pdm09 viruses, the majority of 6B viruses including those in the emerging sub-subgroups, remain antigenically1 closely related to the vaccine virus. In addition, a pool of human post-vaccination sera collected from healthy adults in the United States of America who received influenza vaccine in the 2015-2016 season well inhibited all recent viruses tested in the WHO CC at the Centers for Disease Control and Prevention (CDC) in Atlanta.
Influenza vaccines containing an A/California/7/2009-like component, as recommended by WHO for use in the 2015-2016 seasonal vaccines, are expected to provide good protection against infections associated with the currently circulating A(H1N1)pdm09 virus. WHO strongly recommends that high risk groups be vaccinated against influenza (Weekly Epidemiological Record, 23 November 2012, vol. 87, 47 (pp. 461-476)). Risk groups for influenza include people at increased risk of exposure to influenza virus as well as those at particular risk of developing severe disease resulting in hospitalization or even death. The former group includes healthcare workers (HCWs) whereas the latter groups include pregnant women, children aged 6–59 months, the elderly and individuals with specific chronic medical conditions.
Globally more than 450 A(H1N1)pdm09 viruses have been tested for antiviral susceptibility to neuraminidase inhibitors, of which only two viruses showed reduced susceptibility. Currently circulating viruses are expected to be susceptible to the antiviral drugs oseltamivir and zanamivir. Early administration of neuraminidase inhibitors, ideally within 48 hours of influenza symptom onset, reduces severe complications and death and is recommended for persons at increased risk and progressive disease. When influenza is suspected in such populations, antiviral treatment should not wait for diagnostic confirmation but should start immediately.
Countries are encouraged to continue surveillance and programmatic disease control activities, and to timely share surveillance information with WHO and representative viruses with WHO CCs of GISRS in order to have continuous risk assessment of circulating and emerging influenza viruses.