#11,810
The World Health Organization has published their (semi) monthly Influenza at the Human-Animal Interface risk assessment (dated Oct 3rd, but just posted) which provides details on fivew different types of human novel flu (H5N6, H7N9, H3N2v, H1N2v, and H9N2) infections reported since the July 19th update.
First their overview, then we'll take a closer look at the individual reports.
Influenza at the human-animal interface
Summary and assessment, 20 July to 3 October 2016
- New infections: Since the previous update, new human infections with A(H5N1), A(H7N9),A(H9N2), A(H1N2)v and A(H3N2)v viruses were reported.
- Risk assessment: The overall public health risk from currently known influenza viruses at the human-animal interface has not changed. Further human infections with viruses of animal origin can be expected, but the likelihood of sustained human-to-human transmission remains low.
- Risk management: Status of the development of candidate vaccine viruses (CVVs) and selection of new CVVs of zoonotic influenza for influenza pandemic preparedness purposes were reviewed in a recent WHO consultation.2
- IHR compliance: All human infections caused by a new influenza subtype are reportable under the International Health Regulations (IHR, 2005).3 This includes any animal and non-circulating seasonal viruses. Information from these notifications will continue to inform risk assessments for influenza at the human-animal interface.
Note: We looked at the latest CVV consultation mentioned in the Risk Management section a week ago in WHO: Updated Candidate Vaccine Viruses For Pandemic Preparedness.
The first section, on H5N1, provides some detail on the two new cases reported by Egypt I blogged on earlier today (see WHO EMRO:H5N1 Update In Egypt - September 2016).
Since the last update, two new laboratory-confirmed human cases of avian influenza A(H5N1) virus infection were reported to WHO. A 3-year-old male resident of Fayoum Governorate, Egypt, had onset of symptoms on 7 May 2016, was hospitalized and treated with antivirals for pneumonia, but passed away on 20 May 2016. Prior to his illness, the case was exposed to domestic poultry that appeared healthy.
The second case was a 3-year-old female resident of Giza Governorate, Egypt, who had onset of symptoms on 24 July 2016, was hospitalized and treated with antivirals for pneumonia, but passed away on 31 July 2016. Prior to her illness, the case was exposed to poultry purchased from a market that later died.
Investigation and follow up of contacts of the two cases took place for 14 days with no further cases detected. Avian influenza A(H5N1) viruses are enzootic in poultry in Egypt.
Since 2003, a total of 856 laboratory-confirmed cases of human infection with avian influenza A(H5N1) virus, including 452 deaths, have been reported to WHO from 16 countries
Although other influenza A(H5) subtype viruses have the potential to cause disease in humans, no human cases, other than those with influenza A(H5N1) and A(H5N6), have been reported so far. According to reports received by the World Organisation for Animal Health (OIE), various influenza A(H5) subtypes continue to be detected in birds in West Africa, Europe and Asia.
The A(H5N1) virus outbreaks in poultry in West Africa continue since 2014 with a newly-affected country, Togo, now reporting outbreaks. No human infections associated with these outbreaks in Western and Central Africa have been identified to date, despite surveillance and testing of human samples.
Influenza A(H5) viruses are highly diverse and continue to evolve. Further details about these viruses can be found in the September 2016 WHO report of the antigenic and genetic characteristics of zoonotic influenza viruses, including the development of two new candidate vaccine viruses for pandemic preparedness
The report on H7N9 covers 5 cases previously reported by WHO (see HK CHP: Additional Details On China's July H7N9 Cases).
Avian influenza A(H7N9) viruses
Current situation:
During this reporting period, China reported five laboratory-confirmed human cases of A(H7N9) virus infection to WHO on 11 August 2016, including one fatal case. One cluster of three cases was reported for which the possibility of human-to-human transmission for two cases in the cluster cannot be excluded. For more details on these cases, see Table 1 below and the Disease Outbreak News.
We continue to see an uptick in H9N2 human infections in China, although it isn't certain whether this is due to better testing and surveillance or to an increase in infections.
Avian influenza A(H9N2) viruses
Current situation:
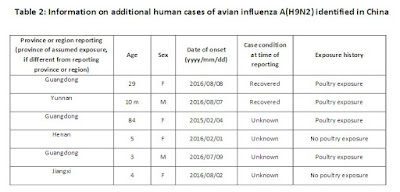
Since the last update10, two laboratory-confirmed human cases of A(H9N2) virus infection were reported to WHO from China. No abnormalities were observed among close contacts of these cases at the time of reporting. In addition, four other cases were identified in China over the past six months, including one retrospectively diagnosed case from 2015. For more details on these cases,see Table 2 below.
The outcome of a previously reported case, a 57-year-old woman with underlying conditions from Sichuan province in China, was recently reported as fatal. This would be the first fatality associated with influenza A(H9N2) virus infections in humans. Avian influenza A(H9N2) viruses are enzootic in poultry in China.
Beyond these avian viruses, today's report also contains information on two swine viruses; H1N2v and H3N2v. The first one, from Brazil, is of particular interest because the patient reported no contact with swine.
Influenza A(H1N2)v viruses
WHO was notified of one new laboratory-confirmed human infection with an A(H1N2) variant virus in Brazil. The infection occurred in an adolescent in November 2015, in the southern region of Brazil, and was detected through surveillance for influenza-like illness. The virus was similar to A(H1N2) swine influenza viruses isolated in 2011 and 2013 from pigs in that region, and the case did report contact with swine prior to illness onset. No further cases were detected at the time, and retrospective analyses of samples collected in the same area have not revealed any additional cases. Genetically, this virus does not resemble the influenza A(H1N2)v viruses detected in the United States this year.
This report also describes the H3N3v outbreak in Michigan and Ohio, involving 18 fair goers with swine exposure, which I blogged extensively on in August (see here, here, and here).
The WHO's Risk assessment for swine flu reads:
Risk Assessment:
1. What is the likelihood that additional human cases of infection with swine influenza viruses will occur? Influenza A(H1N2) and A(H3N2) viruses circulate in swine populations in many regions of the world. Depending on geographic location, the genetic characteristics of these viruses differ. Most human cases are exposed to swine influenza viruses through contact with infected swine or contaminated environments. Human infection tends to result in mild clinical illness. Since these viruses continue to be detected in swine populations, further human cases can be expected.This report concludes with reminders on the importance of global surveillance, epidemiological investigation, and timely reporting of novel flu infections - all required by the 2005 IHR regulations - and yet still not fully implemented or rigorously followed by many governments around the globe.
2. What is the likelihood of human-to-human transmission of swine influenza viruses? No case clusters have been reported. Current evidence suggests that these viruses have not acquired the ability of sustained transmission among humans, thus the likelihood is low.
3. What is the risk of international spread of swine influenza viruses by travellers? Should infected individuals from affected areas travel internationally, their infection may be detected in another country during travel or after arrival. If this were to occur, further community level spread is considered unlikely as these viruses have not acquired the ability to transmit easily among humans.
- Due to the constantly evolving nature of influenza viruses, WHO continues to stress the importance of global surveillance to detect virological, epidemiological and clinical changes associated with circulating influenza viruses that may affect human (or animal) health. Continued vigilance is needed within affected and neighbouring areas to detect infections in animals and humans. As the extent of virus circulation in animals is not clear, epidemiological and virological surveillance and the follow-up of suspected human cases should remain high.
- All human infections caused by a new influenza subtype are notifiable under the International Health Regulations (IHR, 2005).15 State Parties to the IHR (2005) are required to immediately notify WHO of any laboratory-confirmed16 case of a recent human infection caused by an influenza A virus with the potential to cause a pandemic.6 Evidence of illness is not required for this report.
- It is critical that influenza viruses from animals and people are fully characterized in appropriate animal or human health influenza reference laboratories and reported according to international standards. Under WHO’s Pandemic Influenza Preparedness (PIP) Framework, Member States are expected to share their influenza viruses with pandemic potential on a regular and timely basis with the Global Influenza Surveillance and Response System (GISRS), a WHO-coordinated network of public health laboratories. The viruses are used by the public health laboratories to assess the risk of pandemic influenza and to develop candidate vaccine viruses.