R0 (pronounced R-nought) or Basic Reproductive Number.
Essentially, the number of new cases in a susceptible population likely to arise from a single infection. With an R0 below 1.0, a virus (as an outbreak) begins to sputter and dies out. Above 1.0, and an outbreak can have `legs’.
# 8490
Two months ago, in mBio: Spread, Circulation, and Evolution of MERS-CoV, we looked at a study that focused on the evolutionary changes in the MERS coronavirus since its introduction to the human population, and its apparent efficiency in transmitting between humans.
At the time, based on 180 human cases reported over roughly 18 months, the authors determined that the MERS virus had an R0 of less than 1.
In other words, it wasn’t spreading efficiently enough to sustain an ongoing epidemic.
They warned, however, that over time evolutionary pressures could allow the virus to better adapt to human hosts, writing:
MERS-CoV adaptation toward higher rates of sustained human-to-human transmission appears not to have occurred yet. While MERS-CoV transmission currently appears weak, careful monitoring of changes in MERS-CoV genomes and of the MERS epidemic should be maintained. The observation of phylogenetically related MERS-CoV in geographically diverse locations must be taken into account in efforts to identify the animal source and transmission of the virus.
Fast forward 60 days, and suddenly we are seeing at least two large clusters of MERS – one in the UAE (12 cases) and the other in Jeddah, Saudi Arabia (45 cases) – and of particular note, both involve a large number of healthcare workers.
A cohort that, at least in theory, should be practicing stringent infection control protocols.
While we don’t have the specifics on the source of the initial infection or the subsequent chain of transmission in either cluster, their size and duration are at least suggestive of more robust transmission.
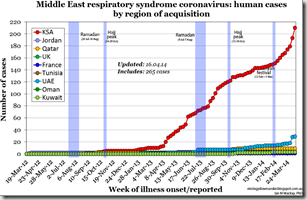
Dr. Ian Mackay’s chart from earlier this week (see below) illustrates this sudden jump in cases counts in KSA and the UAE.
All of which begs the $64 question: Has something changed with the virus?
It is a question raised by Dr. Michael Osterholm – Director of CIDRAP - yesterday (see Osterholm & Mackay On MERS), and one that has been on the minds of many watching the evolution of these two large clusters.
Definitive answers to that question may be some time in coming, as it will require detailed genetic analysis and an in-depth epidemiological investigation to establish the facts. It isn’t, however, the only possible explanation.
Another possibility is that we are seeing a couple of `super spreader’ events, reminiscent of what was seen in Al-Hasa a year ago (more on that later).
During the SARS epidemic of 2003, we know that transmission of that coronavirus was typically fairly inefficient.
An infected person might only infect 1 or 2 additional people, and sometimes none. But a small percentage of those infected were far more efficient in spreading the disease, with some responsible for 10 or more secondary infections.
This super spreader phenomenon gave rise to the 20/80 rule, that 20% of the cases were responsible for 80% of the transmission of the virus (see 2011 IJID study Super-spreaders in infectious diseases)
Last year, for the 10 year anniversary of the SARS epidemic, the CDC authored a review of the outbreak called Remembering SARS: A Deadly Puzzle and the Efforts to Solve It. While the whole article is a good read, I’ve lifted some excerpts from the section entitled: Solving the Mystery of “Super Spreaders”.
In the 2003 outbreak, in some instances outside the United States, a single SARS patient infected large numbers of people. At the same time, other patients did not infect people who came in contact with them.
Researchers found that the virus was typically spread from person to person by large droplets (less efficient spread because it would be too big to linger in the air); however, at other times, clusters of illness suggested aerosol spread (where the virus can linger in the air longer after an ill person coughs) causing more spread of infections from a single sick person.
CDC investigated the so-called “super spreaders.” They wanted to know if there were differences in when and for how long people ill with SARS might shed the virus, making them contagious to others. In the past, super spreaders had been documented during other disease outbreaks such as rubella, tuberculosis and Ebola. A common feature of super spreaders was that hospitals served as a source for the disease to widely infect others.
Last summer, in Branswell:The NEJM Saudi MERS-CoV Cluster Report, we looked at a review of the hospital associated cluster involving 23 cases in the Al-Hasa region, occurring between April 1st and May 23rd.
Helen Branswell’s report, which is still online, discussed the `super spreader’ angle.
Saudi MERS outbreak showed SARS-like features, including possible superspreader
Helen Branswell, The Canadian Press Jun 19, 2013 05:00:17 PM
TORONTO – A long-awaited report on a large and possibly still ongoing outbreak of MERS coronavirus in Saudi Arabia reveals the virus spreads easily within hospitals, at one point passing in a person-to-person chain that encompassed at least five generations of spread.
The study, co-written by Toronto SARS expert Dr. Allison McGeer, also hints there may have been a superspreader in this outbreak, with one person infecting at least seven others.
As was common with SARS, and featured in the Al-Hasa report above, we are once again seeing the familiar pattern of unusually large clusters centered around health care facilities.
Whether they signify an evolutionary change in the virus, the effects of `super spreaders’, or a combination of both - or perhaps some other dynamic - is impossible to tell at this point.
All we can say right now is that the pattern of disease spread appears – at least temporarily, and in these two locations - to have changed in recent weeks, and that it bears watching.
As Dr. Osterholm said yesterday, we are definitely in a `stay tuned’ moment.