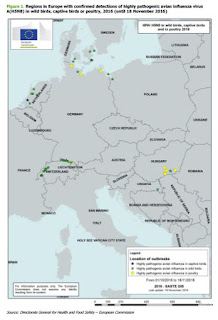
 |
| Credit ECDC RRA |
#11,922
Earlier today we looked at the WHO: Assessment Of Risk Associated With HPAI H5N8, which is now joined by a detailed RRA from the ECDC on the risks to Europe posed by the H5N8 virus.
As we've seen previously from the WHO and the CDC, the ECDC says the risks of zoonotic transmission to the general public is considered to be very low.
Those actively involved in culling of birds are at somewhat greater risk, and should use protective gear and receive oseltamivir prophylaxis for up to 10 days after the last contact. Given H5N8's history of reassorting with other viruses, it is always possible the threat could change over time.
The full report is well worth reading, as it provides a good deal of background - not only on the virus in Europe - but its expansion from Asia two winters ago. Below you'll find excerpts from their conclusions.
ECDC threat assessment for the EU
To date, no human infections with this virus have been reported and the risk of zoonotic transmission to the general public in EU/EEA countries is considered to be very low.
However, this second HPAI A(H5N8) introduction event is another indication of the widespread circulation and continuous re-assortment of avian influenza viruses, notably for H5 viruses in animal populations, which continues to pose a long-term risk of human influenza pandemics. The recent emergence of A(H5N6) viruses in China that have caused severe disease in humans highlights the possibility of transmission from birds to humans of reassorted viruses from clade 2.3.4.4 [21]. The full genome sequences of several recent HPAI A(H5N8) viruses showed that these viruses to date are still essentially bird viruses without any specific increased affinity for humans.
Strict food safety and veterinary measures are in place in the EU/EEA Member States to prevent meat or eggs from unhealthy animals entering the food chain. Even in the unlikely event of the virus being present in meat or eggs sold in the EU, thorough cooking destroys the virus, so well-cooked meat and eggs pose no risk. A(H5N8) viruses are reliably detectable using EU-recommended avian influenza laboratory methods (M 1.2 and H5), which are continuously reviewed by the Avian influenza EU Reference Laboratory as being fit for purpose to support outbreak and surveillance investigations. Any modifications to existing assays must be balanced against broad sensitivity of such assays for application in wider passive and active surveillance programmes where the virus subtype(s) is unknown.
It is suggested that laboratory methods in human national influenza reference laboratories be evaluated for their suitability to detect A(H5N8) viruses.
As a precautionary principle and based on experience and the risk posed by A(H5N1) viruses, several countries in the EU apply preventative measures to lower the possible risk of human exposure and any subsequent infection due to exposure to avian influenza viruses.
In order to prevent spread of avian influenza viruses, Directive 2005/94/EC [23] requires that Member States have contingency plans detailing measures for the killing and safe disposal of infected poultry, feed and contaminated equipment as well as procedures and methods for cleaning and disinfection.
Reinforcing biosecurity measures to prevent contact between domestic poultry and wild birds is expected to reduce the risk of infection if wild birds are identified as a source of infection.
The Directive also requires the development of contingency plans for the control of avian influenza in poultry in collaboration with public health and occupational health authorities to ensure that persons at risk are sufficiently protected from infection. Personal protective equipment, and in particular respiratory protection, should be considered. Persons at risk of exposure to the virus are mainly those in direct contact with or handling diseased birds and poultry, or their carcasses (e.g. farmers, hunters, veterinarians and labourers involved in the culling).
Protective measures have been recommended in accordance with national guidelines for HPAI, including personal protection equipment and oseltamivir prophylaxis for up to 10 days after the last contact [29]. Persons exposed to the virus are asked to report any symptoms to the municipal health service and in the event that they develop conjunctivitis or influenza-like-illness, sampling material will be obtained for diagnostic testing. These measures have also been recommended by the US Centers for Disease Control and Prevention (CDC) during HPAI A(H5N1), A(H5N2) and A(H5N8) outbreaks in the US in 2015 [30]. The FAO also raised awareness regarding HPAI A(H5N8) providing general recommendations as well as those pertaining to poultry producers, hunters and national authorities [31].
Routine vaccination with seasonal influenza vaccine is recommended in most countries for workers having contact with birds and poultry to minimise the possibility of co-infection with human and avian influenza viruses thereby reducing the risk of reassortment.
Persons in direct contact with infected poultry before or during culling and disposal, including poultry workers, should be monitored for 10 days, in order to identify possible related influenza-like symptoms, fever or conjunctivitis. Local health authorities may consider actively monitoring these groups. Administration of antiviral prophylaxis for exposed persons (as recommended for A(H5N1)) can be considered as a precautionary measure depending on the local risk assessment (i.e. intensity of exposure) and in the context of the start of seasonal influenza in the EU to minimise the likelihood of reassortment events [32]. Healthcare workers managing symptomatic exposed (or possible) cases should follow standard, contact and respiratory precautions, depending on the local risk assessment.
(Continue . . . .)