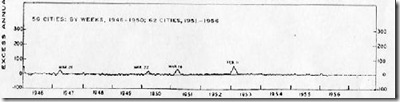
#13,022
The nearly 20 year roller-coaster of flu season P&I (pneumonia and influenza) mortality figures (see above) - lifted and stitched together from multiple CDC's FluView reports - illustrates nicely the variability in intensity of flu seasons in the United States since 1999.
Some non-pandemic seasons - such as 1999-2000, 2003-2004, 2007-2008, 2012-2013 and 2014-2015 - are clearly rougher than others (like 2000-2001, 2002-2003, and 2011-2012), when P&I mortality barely reached the epidemic threshold.While warnings go out every year that flu can be deadly and that it claims hundreds of thousands of lives every year (see The Lancet: Estimates Of Global Seasonal Flu Respiratory Mortality, this year we appear on track for a particularly rough season across North America.
The predominant strain is H3N2, which hits the elderly particularly hard and against which the vaccine is expected to provide only modest protection, and the season has started early.
While we appear to have all of the ingredients in place for the makings of a bad flu season, it is worth remembering what happened during the winter of 1951 - starting in Liverpool, England - that came essentially without warning.Across the Northern Hemisphere, the winter of 1950-1951 had been an average flu year, with the dominant flu called the `Scandinavian strain', which produced mild illness in most of its victims. In fact, if you look at a graph of flu activity for the United States, running from 1945 to 1956, you'll see nary a blip.
But in December of 1950 a new strain of virulent influenza appeared in Liverpool, England, and by late spring, had spread across much of England, Wales, and Canada. While we've looked at this event before, it has been a few years, and today seems a good day to revisit the impact of rogue flu years.
The following comes from an absolutely fascinating EID Journal article: Viboud C, Tam T, Fleming D, Miller MA, Simonsen L. 1951 influenza epidemic, England and Wales, Canada, and the United States.
The 1951 influenza epidemic (A/H1N1) caused an unusually high death toll in England; in particular, weekly deaths in Liverpool even surpassed those of the 1918 pandemic. . . . . Why this epidemic was so severe in some areas but not others remains unknown and highlights major gaps in our understanding of interpandemic influenza.According to this study, the effects on the city of origin, Liverpool, were horrendous.
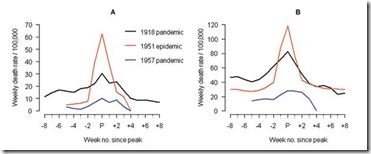
In Liverpool, where the epidemic was said to originate, it was "the cause of the highest weekly death toll, apart from aerial bombardment, in the city's vital statistics records, since the great cholera epidemic of 1849" (5). This weekly death toll even surpassed that of the 1918 influenza pandemic (Figure 1)
This extraordinary graph shows the excess deaths in Liverpool during this outbreak (red line), while the black line shows the peak deaths during the 1918 pandemic. This chart shows excess deaths by A) respiratory causes (pneumonia, influenza and bronchitis) and B) all causes.
For roughly 5 weeks Liverpool saw an incredible spike in deaths due to this new influenza. And it didn’t just affect Liverpool. While it appears not to have spread as easily as the dominant Scandinavian strain, it managed to infect large areas of England, Wales, and Canada over the ensuing months.
The authors of this study describe the spread of this new influenza:
Geographic and Temporal Spread
Influenza activity started to increase in Liverpool, England, in late December 1950 (5,13). The weekly death rate reached a peak in mid-January 1951 that was ≈40% higher than the peak of the 1918–19 pandemic, reflecting a rapid and unprecedented increase in deaths, which lasted for ≈5 weeks [5 ] and Figure 1).
Since the early 20th century, the geographic spread of influenza could be followed across England from the weekly influenza mortality statistics in the country's largest cities, which represented half of the British population (13). During January 1951, the epidemic spread within 2 to 3 weeks from Liverpool throughout the rest of the country.
For Canada, the first report of influenza illness came the third week of January from Grand Falls, Newfoundland (19). Within a week, the epidemic had reached the eastern provinces, and influenza subsequently spread rapidly westward (19).
For the United States, substantial increases in influenza illness and excess deaths were reported in New England from February to April 1951, at a level unprecedented since the severe 1943-44 influenza season. Much milder epidemics occurred later in the spring elsewhere in the country (9).
Perhaps most telling is this graph showing the death rates in England and Wales between 1950 and 1971, which incorporates the no-name outbreak of 1951, and the 1957 and 1968 pandemics. As you can see, the 1951 event was more severe than either of the two `official' pandemics.
Crude death rates (blue bars), death rates adjusted for summer trends in mortality unrelated to influenza (pink bars).
Getting started relatively late in the flu season, this new strain never managed to spread much beyond UK and Eastern Canada. Nor did it reappear the following flu season. It simply vanished as mysteriously as it appeared.
A good thing, considering its virulence. A return the following year could have sparked a global pandemic.As far as I know, there are no isolates or genetic sequences available for this rogue flu virus, and so the reasons behind its unusual virulence remain a mystery.
While most striking, 1951 isn't the only pseudo-pandemic year in the 20th century. The second one - which struck while I was an impossibly young paramedic back in the mid-1970s - was the so-called `Russian Flu' of 1977.
From 1918 through 1976 we’d seen three influenza A pandemics (1918, 1957, 1968) and each time the pandemic virus completely supplanted the previously circulating influenza A virus. The descendents of the 1918 H1N1 virus reigned supreme (and alone) until it was unseated by H2N2 in 1957. H2N2 held court until H3N2 pushed it aside eleven years later.
There were certainly influenza B viruses in the mix, but the norm appeared to be only one influenza A subtype each flu season.In 1977, however, a second influenza A virus abruptly appeared, or rather - re-appeared – and began circulating along side H3N2. A throwback to the 1950s, the 1977 H1N1 `Russian flu’ was generally mild in adults, but hit children and adolescents born before 1957 the hardest.
Since 1977 we’ve seen two influenza A subtypes co-circulate every year. Even when the 2009 H1N1 pandemic virus completely supplanted the old (1977) H1N1 virus, H3N2 continued to circulate.For years scientists have tried to explain how a virus – gone from the wild for 20 years – could just suddenly reappear. Particularly one that was practically identical to a strain last seen more than a quarter of a century before.
The most popular (and plausible) theory has been that of an accidental release from a Russian or Chinese research facility, but solid evidence remains lacking (see mBio The Reemergent 1977 H1N1 Strain and the Gain-of-Function Debate.)While we wait, and watch carefully, for signs of a novel flu making the jump to humans, it is important to remember that seasonal flu can increase in virulence, transmissibility, or overall impact with little or no warning.
Sometimes it is for reasons we can see coming, such as when a virus `drifts' away from the current vaccine over the summer (see CDC HAN Advisory On `Drifted’ H3N2 Seasonal Flu Virus).
Other times it can be due to an unpredictable mutation that increases virulence, such as D222G which is linked to deeper lung infections and greater virulence, which we saw described during the 2009 H1N1 pandemic.
This relatively rare amino acid substitution at position 222 (225 using H3 numbering) from aspartic acid (D) to glycine (G) allows the virus to bind to receptors found deeper in the lungs, and is linked to the development of more severe pneumonia.Since 2009 we've seen this mutation appear sporadically among H1N1 viruses in Russia, India, and the United States (see EID Journal: Emergence of D225G Variant A/H1N1, 2013–14 Flu Season, Florida.
The good news, at least with D222G, is that its incidence has been low ( < 2%) and a 2013 study in EuroSurveillance: Revisiting The D222G Mutation In A/H1N1pdm09, suggested viruses with this mutation don’t transmit well in the wild, and that most of the time this variant comes about through a spontaneous mutation in the host after the host has been infected.We have just entered the 100th anniversary year of the Great Influenza Pandemic of 1918, and a good deal will justifiably be written and said about that catastrophic event over the next 12 months. I'm sure I'll be contributing my share in this blog.
But it is also important to remember that `no-name' interpandemic flu seasons can kill tens . . . sometimes hundreds . . . of thousands of people in a matter of just a few months.Influenza & Pneumonia are listed as the 8th leading cause of death in the United States, and there are studies to suggest that ranking is low, as it only counts deaths directly attributed to respiratory infections.
Last year, in Int. Med. J.: Triggering Of Acute M.I. By Respiratory Infection we looked at research from the University of Sydney that found the risk of a heart attack is increased 17-fold in the week following a respiratory infection such as influenza or pneumonia.Given its impact, and unpredictability, I'm continually amazed that seasonal influenza isn't taken as a serious health threat by the public.
Many people sitll go to work, or school, sick. Too many don't cover their coughs and sneezes in public, or use hand sanitizer. And many eschew the vaccine.Hopefully it won't take another `Liverpool flu'-like event for seasonal influenza to gain the street creds it truly deserves.