Credit ACIP/CDC
#15,911
Although the number of cases of CVST (Cerebral Venous Sinus Thrombosis) with Thrombocytopenia remains exceedingly small (6 cases out of 6+ million vaccine recipients), on Tuesday the CDC & FDA Recommended a `Pause' On J&J Vaccine Administration Over Blood Clot Concerns, and yesterday, the CDC's ACIP (Advisory Committee on Immunization Practices) recommended extending that `pause' another 7 to 10 days (see CIDRAP report) to gather more information.
These webinars are often technical, and are of greatest interest to clinicians and healthcare providers, but also may be of interest to the general public. As always, If you are unable to attend the live presentation, these (and past) webinars are archived and available for later viewing at this LINK.On Tuesday, the CDC also released a HAN ALERT, alerting clinicians of the risk, and informing them how to report suspected cases. Later today (April 15th), the CDC will hold a hastily called COCA Call in order to provide more details to clinicians.
First details on today's COCA call, then I'll return with a postscript.
Johnson & Johnson/Janssen COVID-19 Vaccine and Cerebral Venous Sinus Thrombosis with Thrombocytopenia – Update for Clinicians on Early Detection and Treatment
Overview
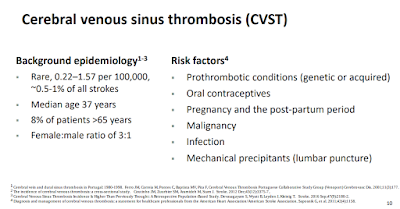
This COCA Call will present the latest evidence on cerebral venous sinus thrombosis (CVST) with thrombocytopenia associated with the administration of the Johnson & Johnson/Janssen COVID-19 vaccine. Speakers will discuss what is known about CVST, the importance of early detection, and updated vaccine recommendations.
Presenters
Sara Oliver, MD
LCDR, U.S. Public Health Service
Co-lead, Advisory Committee for Immunization Practices COVID-19 Vaccines Work Group
COVID-19 Response
Centers for Disease Control and Prevention
Tom Shimabukuro, MD, MPH, MBA
CAPT, U.S. Public Health Service
Vaccine Safety Team Lead
COVID-19 Response
Centers for Disease Control and Prevention
Call Materials
None at this time
Call Details
When:
Thursday, April 15, 2021,
2:00 PM – 3:00 PM ET
Webinar Link:
https://www.zoomgov.com/j/1614336614?pwd=ZVhQUHoyaG4zVFdua2czcE9EU20wUT09external icon
Passcode: 160026
Dial In:
US: +1 669 254 5252
or +1 646 828 7666
or +1 551 285 1373
or +1 669 216 1590
International numbersexternal icon
iPhone one-tap:
US: +16692545252,,1614336614#,,,,*160026# or +16468287666,,1614336614#,,,,*160026#
Webinar ID: 161 433 6614 Add to Calendar
The negative publicity surrounding both the J&J and AstraZeneca vaccines the past few weeks (see WHO statement on AstraZeneca COVID-19 vaccine safety signals), has led to moratoriums and restrictions on their use in some countries (see Denmark's SSI Statement), and yesterday major media reported that EU Commission to end AstraZeneca and J&J vaccine contracts at expiry - paper.
The number of reported cases (n=6) connected to the J&J vaccine is well within the expected `background range' for these admittedly rare thrombotic events (see below), so these recommended pauses have raised the eyebrows - and in some cases the ire - of researchers who believe the benefits of both theJ&J and AstraZeneca vaccines far outweigh the risks.
And on a purely actuarial basis, that is likely true. Even if these vaccines are causing these blood clot complications (which has not been established), these vaccines would undoubtedly still save more lives than they cost, even at a much higher rate of occurrence.
But with the public's faith in the safety of these vaccines already shaken, were the government to appear to be plunging callously ahead despite their concerns, vaccine hesitancy could quickly spread to the other vaccines and stall the vaccination process before the pandemic can be controlled.
The CDC, ACIP, WHO, EU Commission, and public health in general find themselves in a difficult position here, as they not only need to make sure they are right about the risks, they must then `sell' any decision to continue with the J&J or AZ vaccines to the general public.
And right now, that could be hard sell.
The good news is that the the pause on J&J's vaccine will have little impact on the vaccine campaign in the United States over the next couple of weeks. Other nations (like Demark) who ordered J&J to replace their AstraZeneca vaccines are in more of a bind.
Hopefully, a week or 10 days from now, we'll have more data and better clarity on the situation.