#16,159
Due to the COVID pandemic - and our interventions against it - seasonal flu has practically disappeared globally over the past 18 months (see MMWR: Changes in Influenza and Other Respiratory Virus Activity During the COVID-19 Pandemic), which has likely left us all with a bit of an `immunity deficit' against influenza.
Flu will undoubtedly return, but whether that happens this year - potentially as a `twindemic' with COVID - or later, remains to be seen. But there are concerns that flu could be unusually severe when it does return (see UK Academy Of Medical Sciences: Looking Ahead To COVID-19 Over Winter 2021/22 & Beyond).
A particularly bad flu season could kill between 80,000 and 100,000 Americans (see 2018's CDC: More Than 900,000 Hospitalizations & 80,000 Deaths In Last Winter's Flu Season), even without the exacerbation of COVID.
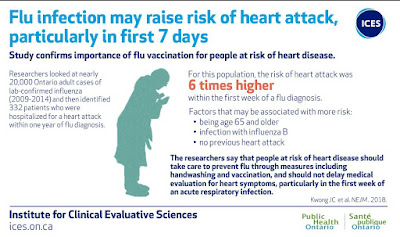
Most years flu vaccines are moderately protective against influenza infection, and they can often reduce the severity of illness even when they don't prevent infection. Since influenza is also linked to increased risk of heart attack and stroke, getting the vaccine may also help prevent these devastating outcomes.
But vaccinated or not, if you get influenza, there are still pharmaceutical treatments that can reduce the duration, and severity, of your infection. Assuming you take them early.
Unfortunately, their uptake is far less than optimal, driven in part by a lack of awareness (on the part of both the public and some doctors), and due to negative and misleading tabloid style reporting (see The Conversation: The Rise & Fall Of The Challenge To Tamiflu) and social media posts.
Each fall we see a push by the CDC to promote more seasonal flu vaccine uptake, and to encourage the use of antivirals to reduce the severity of influenza infection. This year, with the spectre of a `twindemic' of COVID and flu threatening to overwhelm hospitals, getting this message out is probably more important than ever.
Yesterday the CDC published a summary of a new study, published in the journal Pediatrics, which shows the benefits of early use of antivirals in children hospitalized with influenza. First the summary, then a link, and the abstract to the study.
Early flu antiviral treatment can shorten hospital stays in children with flu, new CDC study shows
Español | Other Languages
September 1, 2021 – A new CDC study shows that early flu antiviral treatment was associated with significantly shorter hospitalizations in children with laboratory-confirmed flu and higher-risk medical conditions or children with laboratory-confirmed flu in an intensive care unit (ICU).
Compared with those not receiving flu antiviral drugs, length of stay was shorter for children who were treated within 2 days of illness onset, with antiviral treatment increasing the probability of hospital discharge by:
- 37% per day for hospitalized children with underlying medical conditions,
- and 46% per day for children in the ICU.
For children with underlying medical conditions, median length of hospital stay was 2 days for those who received early flu antiviral treatment (within 2 days of illness onset) compared to 3 days for those not treated with antivirals.
The study included 608 children hospitalized with laboratory-confirmed flu identified through the U.S. Influenza Hospitalization Surveillance Network (FluSurv-NET). The children were grouped into two cohorts:
- The first cohort included 309 children with at least one underlying medical condition not admitted to the intensive care unit (ICU).
- The second cohort included 299 children admitted to an ICU with and without underlying conditions.
The children were treated almost exclusively with oseltamivir.
Influenza causes thousands of hospitalizations and deaths every season in the United States, with children representing a substantial portion of those hospitalized with flu. Antiviral medications are an important adjunct to flu vaccine in the control of flu.
Antiviral treatment is already recommended for hospitalized patients with suspected and confirmed flu. However, evidence for this recommendation in children was previously limited. This study serves to strengthen the body of evidence behind the recommendation for early antiviral treatment in children who are hospitalized with flu.
There are four U.S. Food and Drug Administration (FDA) approved flu antiviral drugs that are recommended by CDC for use in children this flu season:
Families of children at higher risk for serious flu complications should seek care early in the course of flu illness. Your child’s health care provider can help decide whether your child should take antiviral drugs if they become sick with flu this season. Flu signs and symptoms include fever, headache, extreme tiredness, dry cough, sore throat, runny or stuffy nose and muscle aches. It’s important to note that some children with flu will not have a fever.
- Oseltamivir (available as a generic version or under the trade name Tamiflu®) is approved for treatment of flu in children 2 weeks old or older. Oral oseltamivir comes in the form of pills and liquid. Although not part of the FDA-approved indications, use of oral oseltamivir for treatment of flu in infants younger than 14 days old.
- Zanamivir (trade name Relenza®) is approved for treatment of flu in children 7 years and older. It is not recommended for use in children with underlying respiratory disease, including those with asthma and other chronic lung diseases. Inhaled zanamivir is given via a special inhaler (Diskhaler®).
- Peramivir (trade name Rapivab®) is given intravenously and recommended for use in children 2 years and older.
- Baloxavir (trade name Xofluza®) is a pill that is given as a single dose by mouth and is approved for early outpatient treatment of children with flu who are aged 12 years and older.
For more information about flu antivirals and children, visit https://www.cdc.gov/flu/highrisk/children-antiviral.htm.
The link to the study (alas, behind a paywall).
Influenza Antiviral Treatment and Length of StayAngela P. Campbell, Jerome I. Tokars, Sue Reynolds, Shikha Garg, Pam Daily Kirley, Lisa Miller, Kimberly Yousey-Hindes, Evan J. Anderson, Oluwakemi Oni, Maya Monroe, Sue Kim, Ruth Lynfield, Chad Smelser, Alison T. Muse, Christina Felsen, Laurie M. Billing, Ann Thomas, Elizabeth Mermel, Mary Lou Lindegren, William Schaffner, Andrea Price and Alicia M. Fry
Pediatrics September 2021, e2021050417; DOI: https://doi.org/10.1542/peds.2021-050417
Abstract
BACKGROUND Antiviral treatment is recommended for hospitalized patients with suspected and confirmed influenza, but evidence is limited among children. We evaluated the effect of antiviral treatment on hospital length of stay (LOS) among children hospitalized with influenza.
METHODS We included children <18 years hospitalized with laboratory-confirmed influenza in the US Influenza Hospitalization Surveillance Network. We collected data for 2 cohorts: 1 with underlying medical conditions not admitted to the ICU (n = 309, 2012–2013) and an ICU cohort (including children with and without underlying conditions; n = 299, 2010–2011 to 2012–2013). We used a Cox model with antiviral receipt as a time-dependent variable to estimate hazard of discharge and a Kaplan–Meier survival analysis to determine LOS.
RESULTS Compared with those not receiving antiviral agents, LOS was shorter for those treated ≤2 days after illness onset in both the medical conditions (adjusted hazard ratio: 1.37, P = .02) and ICU (adjusted hazard ratio: 1.46, P = .007) cohorts, corresponding to 37% and 46% increases in daily discharge probability, respectively. Treatment ≥3 days after illness onset had no significant effect in either cohort. In the medical conditions cohort, median LOS was 3 days for those not treated versus 2 days for those treated ≤2 days after symptom onset (P = .005).
CONCLUSIONS Early antiviral treatment was associated with significantly shorter hospitalizations in children with laboratory-confirmed influenza and high-risk medical conditions or children treated in the ICU. These results support Centers for Disease Control and Prevention recommendations for prompt empiric antiviral treatment in hospitalized patients with suspected or confirmed influenza.
Getting in to see your healthcare provider within the 1st 48 hours of flu-like symptoms may be difficult, but this year - given the precautions against COVID - doctors may be more willing to call in an Rx for antivirals based on just a phone consult.
It wouldn't hurt to talk to your family doctor now - before flu season begins - to find out how they will handle antiviral prescriptions this winter.