#16,746
With the very strong caveat that reduced testing and reporting by countries around the world have likely significantly impacted the numbers, the WHO reports that the number of COVID cases and deaths have continued to decline since late March. With every recent update, the WHO has advised:
These trends should be interpreted with caution as several countries have been progressively changing COVID--19 testing strategies, resulting in lower overall numbers of tests performed and consequently lower numbers of cases detected.
How long this respite will last is anyone's guess.
This week's WHO update also includes a summary of Vaccine Effectiveness (VE) studies against the Omicron variant, which we'll look at following the global overview.
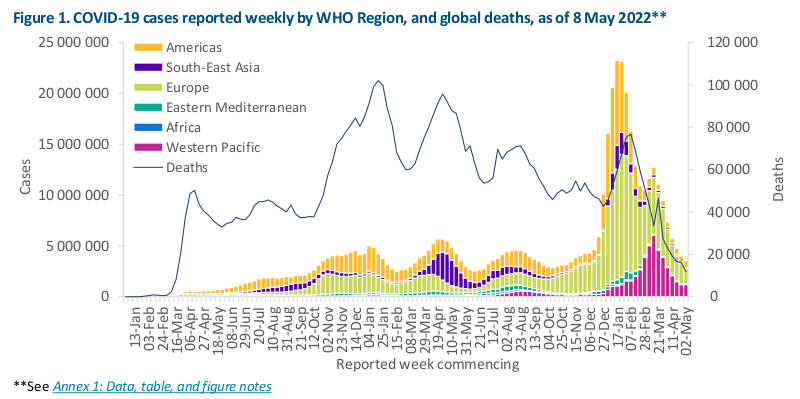
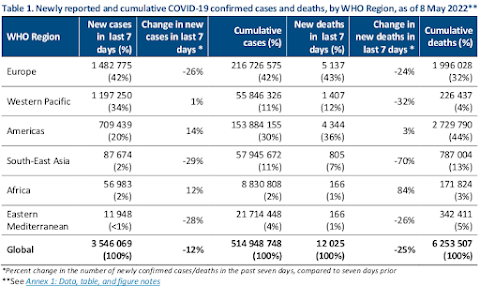
Data as of 8 May 2022Globally, the number of new COVID-19 cases and deaths has continued to decline since the end of March 2022. During the week of 2 through 8 May 2022, over 3.5 million cases and over 12 000 deaths were reported, decreases of 12% and 25% respectively, as compared to the previous week (Figure 1).At the regional level, the number of new weekly cases increased in the Region of the Americas (+14%) and in the African Region (+12%), remained stable in the Western Pacific Region (+1%), and decreased in the remaining three regions. The number of new weekly deaths increased in the African Region (+84%), remained stable in the Region of the Americas (+3%), while decreasing trends were reported in the other four regions.As of 8 May 2022, over 514 million confirmed cases and over six million deaths have been reported globally.
Not unexpectedly, the COVID vaccines developed against the original `wild-type' COVID aren't as effective against the new newer Omicron variants, although they (and their boosters) still provide significant protection against severe illness and death, although their VE wanes over time.
To date, 23 studies from ten countries (Brazil, Canada, Czech Republic, Denmark, Finland, Israel, Qatar, South Africa, the United Kingdom and the United States of America) have assessed the duration of protection of five vaccines against the Omicron variant (six studies assessed VE of primary series vaccination only, six assessed VE of a booster dose vaccination only, and 11 assessed both). Findings from these studies show than has been observed for other four VOCs.Importantly though, in the majority of studies VE estimates against the Omicron variant remain higher for severe disease. Booster vaccination substantially improves VE for all outcomes and for all combinations of schedules with estimates available, for both primary series and booster vaccination.However, studies that assess VE of booster vaccination beyond six months are needed to evaluate the longer duration of protection.For severe disease, within the first three months of primary series vaccination, seven of 12 (58%) VE estimates for the mRNA vaccines (Moderna-Spikevax and Pfizer BioNTech-Comirnaty) were ≥70%. Of the two studies available for vector vaccines, one reported a VE of <70% for AstraZeneca-Vaxzevria, and the other reported a VE of <50% for Janssen-Ad26.COV2.S. One study available for inactivated vaccines (Sinovac-CoronaVac) reported a VE of 50%.Beyond three months after vaccination, 12 of 27 (44%) VE estimates for the mRNA vaccines were ≥70% while 18 (77%) were ≥50%, one of the 12 (8%) VE estimates for AstraZeneca-Vaxzevria was ≥70% while eight (67%) were ≥50%, and the two available VE estimates for Sinovac-CoronaVac were ≥50%; both estimates for Janssen-Ad26.COV2.S beyond three months of vaccination were <50%.A booster dose improved VE estimates against severe disease in all studies, with only one estimate for Pfizer BioNTech-Comrinaty and one for Janssen-Ad26.COV2.S as the booster dose below 70%, between 14 days and three months of receipt of the booster dose (33 estimates evaluated an mRNA booster, two estimates a booster dose of Janssen-Ad26.COV2.S, and one estimate a booster dose of Sinovac-CoronaVac). At three to six months post mRNA booster, 18 of 20 (90%) estimates showed VE ≥70% (an mRNA vaccine was given as the primary series in 13 of the 20 estimates while AstraZeneca-Vaxzevria and Sinovac-CoronaVac were given as the primary series for six and one of the twenty estimates, respectively).VE estimates against symptomatic disease and infection within the first three months of primary series vaccination tended to be lower than those against severe disease, and VE decreased more substantially over time. For symptomatic disease within the first three months of primary series vaccination, only three of 13 (23%) VE estimates for the mRNA vaccines were ≥70%, and seven (54%) were ≥50%; all the three (100%) VE estimates for AstraZeneca- Vaxzevria and the single estimate for Sinovac (CoronaVac) were below 50%.Beyond three months after vaccination, none of the 28 VE estimates were ≥50% (20 estimates evaluated mRNA vaccines, six evaluated AstraZeneca- Vaxzevria, and two evaluated Sinovac-CoronaVac). An mRNA booster after completion of a primary series of an mRNA vaccine, AstraZeneca-Vaxzevria, or Sinovac-CoronaVac, improved VE estimates against symptomatic disease, with four of 21 (19%) VE estimates ≥70% and 16 (76%) estimates ≥50%, between 14 days and three months post booster. However, booster dose protection declined with time since vaccination, with only one of twelve (8%) available estimates indicating a VE of ≥50% at three to six months following receipt of an mRNA booster dose.Estimates for a booster dose of AstraZeneca-Vaxzevria (one estimate) and Sinovac-CoronaVac (one estimate) three to six months post vaccination indicated VE of <50%. VE estimates against infection showed a similar pattern as those against symptomatic disease.
While nowhere near as protective against the Omicron variants as it was against earlier strains, and despite declining protection over time, most of these vaccines (and the mRNA vaccines in particular) still provided valuable protection against severe disease and death.
This study, however, highlights the problem that China faces, since their Sinovac-Coronavac vaccine provides less protection than many of the other vaccines, and many people who received that vaccine did so more than a year ago.
While the effectiveness of COVID vaccines today isn't near what was advertised in early 2021 (95% VE), any protection beats no protection at all.
Which is why I'll be rolling up my sleeve to get my 2nd booster sometime over the summer.