Credit ACIP/CDC
#16,884
It generally takes 3 or 4 months into each flu season (January or February) before the CDC can produce a preliminary estimate of vaccine effectiveness - and those numbers often change significantly by the following summer - when final numbers are released.
With COVID arguably already in its 4th `season' of 2022 (BA.1, BA.2, BA.2.12.1, and BA.4/5), things are moving so fast that by the time we get preliminary numbers on one variant, the pandemic has moved on to another strain.
On Friday - 3 weeks after BA.4/5 become the dominant COVID strains in the U.S. - the CDC's MMWR published an estimate of the effectiveness of 2, 3, or 4 shots of the mRNA vaccine against BA.1, BA.2, and BA.2.12.1.
While vaccine effectiveness (VE) has plummeted since the lofty 95% protection rates reported against the original virus in early 2021, this study does illustrate that getting a 3rd (or 4th) booster shot can temporarily restore a good portion of that lost effectiveness, even against these newer strains.
The CDC suggests that the current vaccines may even provide some protection against serious illness from BA.5 (which is currently dominant in the United States), but it is no secret that recently the FDA Recommended Adding BA.4/5 Spike Protein To Create A Bivalent COVID Booster Shot to this fall's shot.
Of course, even if that can happen, there are no guarantees that BA.4/5 will still be around. BA.2.75 is currently in the wings, and even it may have come and gone by then.
While we aren't where we'd like to be with the current vaccine, getting boosted significantly increases its protection (at least for a few months) against serious illness and death.
I got my 4th booster in June, and my hope is that it will help protect me until the fall.
First, a media statement from the CDC, followed by a link and a brief excerpt from the MMWR report. I'll have a brief postscript after the break.
New COVID-19 Vaccine Effectiveness Data Showcase Protection Gained by 3rd and 4th Doses
Media Statement
Embargoed Until: Friday, July 15, 2022, 1:00 p.m. ET
Contact: Media Relations
(404) 639-3286
A third and fourth COVID-19 vaccine dose offered substantial protection among adults with healthy immune systems who were eligible to receive them during Omicron variant evolution in early 2022, according to a new MMWR published today. The findings of this study, in conjunction with recently published data showing people infected with BA.2 may also have antibodies that can protect against illness with BA.5, suggest that currently available vaccines may provide protection against serious illness caused by the currently circulating BA.5 variant.
To evaluate effectiveness of 2, 3, and 4 doses of mRNA COVID-19 vaccines (Pfizer-BioNTech or Moderna) among adults with healthy immune systems, experts examined VISION Network data on more than 214,000 emergency department/urgent care visits and more than 58,000 hospitalizations with a COVID-19–like illness diagnosis in 10 U.S. states from mid-December 2021 through mid-June 2022. Study findings show:
- When BA.1 was the predominant variant, vaccine effectiveness (VE) was 61% for two doses against COVID-19-associated hospitalizations; VE increased to between 85%–92% after receipt of a third/booster dose.
- When BA.2/BA.2.12.1 became predominant, vaccine effectiveness with two doses was 24% against COVID-19-associated hospitalizations and increased to 52%–69% after a third/booster dose.
- Patterns were similar for emergency department and urgent care encounters, with lower VE during BA.2/BA.2.12.1 predominance and higher VE with 3 or 4 doses compared to VE with 2 doses.
- Among adults ages 50 years and older during BA.2/BA.2.12.1, vaccine effectiveness against COVID-19–associated hospitalization was 55% more than 4 months after a booster/third dose and increased to 80% more than a week after the fourth dose
COVID-19 vaccines remain our single most important tool to protect people against serious illness, hospitalization, and death. Getting vaccinated now will not prevent you from getting an authorized variant-specific vaccine in the fall or winter when they are recommended for you. Given recent increases in deaths and hospitalizations associated with the BA.5 variant, everyone should stay up to date with recommended COVID-19 vaccinations, including additional booster doses for those who are moderately to severely immunocompromised and adults over 50.
Effectiveness of 2, 3, and 4 COVID-19 mRNA Vaccine Doses Among Immunocompetent Adults During Periods when SARS-CoV-2 Omicron BA.1 and BA.2/BA.2.12.1 Sublineages Predominated — VISION Network, 10 States, December 2021–June 2022
Early Release / July 15, 2022 / 71
Ruth Link-Gelles, PhD1; Matthew E. Levy, PhD2; Manjusha Gaglani, MBBS3,4; Stephanie A. Irving, MHS5; Melissa Stockwell, MD6,7,8; Kristin Dascomb, MD, PhD9; Malini B. DeSilva, MD10; Sarah E. Reese, PhD2; I-Chia Liao, MPH3; Toan C. Ong, PhD11; Shaun J. Grannis, MD12,13; Charlene McEvoy, MD10; Palak Patel, MBBS1; Nicola P. Klein, MD, PhD14; Emily Hartmann, MPP15; Edward Stenehjem, MD9; Karthik Natarajan, PhD8,16; Allison L. Naleway, PhD5; Kempapura Murthy, MBBS3; Suchitra Rao, MBBS11; Brian E. Dixon, PhD12,17; Anupam B. Kharbanda, MD18; Akintunde Akinseye, MSPH2; Monica Dickerson1; Ned Lewis, MPH14; Nancy Grisel, MPP9; Jungmi Han16; Michelle A. Barron, MD11; William F. Fadel, PhD12,17; Margaret M. Dunne, MSc2; Kristin Goddard, MPH14; Julie Arndorfer, MPH9; Deepika Konatham3; Nimish R. Valvi, DrPH, MBBS12; J. C. Currey15; Bruce Fireman, MA14; Chandni Raiyani, MPH3; Ousseny Zerbo, PhD14; Chantel Sloan-Aagard, PhD15,19; Sarah W. Ball, ScD2; Mark G. Thompson, PhD1; Mark W. Tenforde, MD, PhD1 (
Summary
What is already known about this topic?
Little is known about COVID-19 vaccine effectiveness (VE) during the Omicron variant BA.2/BA.2.12.2–predominant period or VE of a fourth COVID-19 vaccine dose in persons aged ≥50 years.
What is added by this report?
VE during the BA.2/BA.2.12.2 period was lower than that during the BA.1 period. A third vaccine dose provided additional protection against moderate and severe COVID-19–associated illness in all age groups, and a fourth dose provided additional protection in eligible adults aged ≥50 years.
What are the implications for public health practice?
Immunocompetent persons should receive recommended COVID-19 booster doses to prevent moderate to severe COVID-19, including a first booster dose for all eligible persons and second dose for adults aged ≥50 years at least 4 months after an initial booster dose. Booster doses should be obtained immediately when persons become eligible.
(SNIP)
VE should continue to be monitored in the setting of newly emerging sublineages and variants, including Omicron sublineages BA.4 and BA.5, which became predominant in the United States in late June 2022. Eligible adults should stay up to date with recommended COVID-19 vaccinations, including a first booster dose for all eligible persons and second booster dose for adults aged ≥50 years. Booster doses should be obtained immediately when persons become eligible.
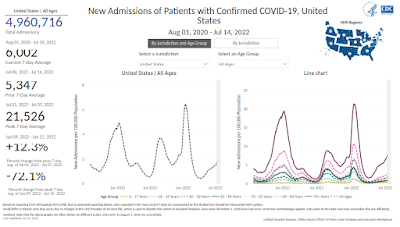
With at-home testing now the norm, and most positive results going unreported, we don't really know how many new COVID infections are occurring in the United States each day. Officially, we are averaging a bit over 125,000 cases a day, but some estimates put that number far closer to a million.
Hospitalizations have been rising steadily since April (see CDC chart below), albeit nowhere near where they were during the peak of the pandemic, suggesting that the vaccine is still providing some protection against severe disease.
With the `next-generation' vaccine unlikely to be available before next October or November, there is renewed talk of extending the eligibility for a 4th booster shot to all adults under the age of 50.
At the rate BA.5 is spreading, however, if it is to have much of an effect, that decision would need to be made sooner rather than later.