#17,360
While SARS-CoV-2 appears to evolve primarily via simple replication errors, it can also reinvent itself by recombination - a process which can occur when a host is infected by 2 or more COVID strains simultaneously (see A COVID Recombination Review).The XBB subvariant is a prime example, as it is recombinant of BA.2.10.1 and BA.2.75 sublineages.
Severely immunocompromised individuals have been linked to long-term carriage of SARS-CoV-2 and the generation of new variants (see SARS-CoV-2 Variants in Patients with Immunosuppression and Persistent COVID-19 infections in immunocompromised people may give rise to variants of concern).
Most of these variants are doomed to becoming evolutionary failures, unable to compete with existing strains. But given enough opportunities, there is always the possibility of seeing a biologically superior variant emerge.
China's CDC Weekly is practically a clone of the U.S. CDC's MMWR, and is published in English, making it easily accessible. This week they describe the detection of a novel variant (an apparent recombinant of BA.5.2.4.48 and BF.7.14) in an immunocompromised patient, who consistently tested positive for COVID while hospitalized over a 6 week period.
First the dispatch, after which I'll return with a brief postscript.
Notes from the Field: The First Case of Co-Infection with Omicron Subvariants BA.5.2.48 and BF.7.14 — Chongqing Municipality, China, February 2023Kun Su1,&;Ying Huang2,&; Xiaofeng Chen2; Fangyuan Liu2; Qi Yan2; Xinyu Jiang2; Jing Xu2; Yongdong Hao2, , ; Jin Yan2, , View author affiliations
On February 14, 2023, a co-infection of Omicron strains was detected in a sample collected and submitted for examination at the Third Affiliated Hospital of Chongqing Medical University.
On February 7, 2023, a 67-year-old female patient living in Yunyang County, Chongqing City, was identified with a history of malignant tumor that had been treated with chemotherapy, radiotherapy, targeting, and other treatments in the past 6 months. Low immunity was suspected, but no other basic diseases, history of smoking, or drinking habits were present. The patient had received two injections of the coronavirus disease 2019 (COVID-19) vaccine (Sinovac Life Sciences Co. Ltd.).
On December 23, 2022, the patient reported poor appetite. On December 29, she developed fatigue, cough, and tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antigen. On January 4, 2023, she experienced chills and fever (temperature of 38.9 ℃) with accompanying cough symptoms. She was admitted to the Traditional Chinese Medicine Hospital of Yunyang County, but did not show significant improvement and was discharged on January 5. On January 6, she was admitted to the Department of Infectious Diseases at the Third Affiliated Hospital of Chongqing Medical University, where she continued to have symptoms of fever and cough. From January 6 to February 12, eight nucleic acid tests were positive in the hospital.
In the investigation, her family members and neighbors were found to be infected in late December, suggesting a potential exposure. During her two hospitalizations, she may have come into contact with other individuals infected with the SARS-CoV-2.
Upper respiratory tract samples were collected from the patients on January 28 and February 7, 2023 and designated as YB20230158 and YB20230202, respectively, to rule out contamination. SARS-CoV-2 whole-genome multiplex polymerase chain reaction (PCR) amplification was performed using the commercial SARS-CoV-2 whole-genome multiplex PCR kits (MicroFuture, Beijing, China). Libraries were prepared using the VAHTS® Universal Plus DNA Library Prep Kit for Illumina (Vazyme Biotech Co.,Ltd.) following the manufacturer’s instructions and sequenced on the NextSeq2000 platform, a combination of metagenomic sequencing and tiling amplicon approaches.
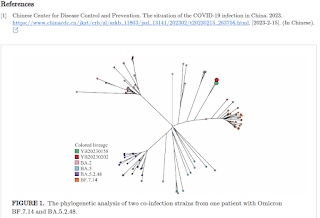
The seqtk-1.3 tool (https://github.com/lh3/seqtk) was used to excise primer sequences and prevent the introduction of mutations caused by primers. The coverage of the two sequencing tests reached 99.7% and 99.8%, respectively, with sequencing depths of 4,142 and 6,551. Phylogenetic analysis (maximum likelihood method) revealed that these two samples formed a separate branch distinct from Omicron subvariants BA.5.2.48 and BF.7.14 (Figure 1).
Mutation sites analysis showed that both samples contained the specific defining sites of Omicron subvariants BA.5.2.48 and BF.7.14, including G1085T, C2710T, C7528T, C8626T, G1085T, C11824T, G12310A, G14181C, C16616A, T17208C, G22599C, T22917G, and G25290T. Verification of the heterozygosity status and frequency of specific mutations in these positions using IGV 2.10.2 (Figure 2) indicated that the patient was simultaneously infected with Omicron subvariants BA.5.2.48 and BF.7.14.
According to the “National Report on the Epidemic of SARS-CoV-2 Infection” released by China CDC, the predominant SARS-CoV-2 strain circulating in Chongqing is BA.5.2.48 (>90%), followed by BF.7.14 (about 3.8%) (1). To date, there have been no reports of co-infection with BA.5.2.48 and BF.7.14 in China, particularly in Chongqing, where the proportion of BF.7.14 is relatively low, making its report more meaningful.Monitoring SARS-CoV-2 variants should be popularized as an important strategy to identify co-infections and recombination cases. As the risk of various variants co-circulating in a region continues to increase, the monitoring of SARS-CoV-2 variants, especially for key populations with immune deficiencies, is becoming increasingly essential.
Online Date: March 17 2023
doi: 10.46234/ccdcw2023.046