#17,526
While viral threats have captured most of our attentions the past few years, we've also been following a number of non-viral threats; including the steep rise in Scarlet Fever and Invasive Group A Streptococcal (IGAS) infections in Europe.Scarlet Fever primarily affects children under the age of 12, although adults can be affected. It is highly contagious, and while there is no vaccine, antibiotics are generally effective when treated early.
Far less common, albeit considerably more serious, is a related illness called iGAS (invasive Group A Strep), which indicates infection of the bloodstream, deep tissues, or lungs, and may result in severe (and frequently fatal) cases of necrotizing fasciitis and streptococcal toxic shock syndrome.
The causative bacterium for both of these infections is Streptococcus pyogenes - which like viruses, can evolve over time – sometimes resulting in increased virulence, greater transmissibility, and/or antibiotic resistance.
Strains are identified by changes in their M-protein gene sequence (emm types) – which often determines virulence - and within these types new variants can emerge.In the middle of the last decade the UK began reporting unusually high numbers of Strep A infections (see The Lancet's Nov 2017 report Resurgence of scarlet fever in England, 2014–16: a population-based surveillance study), and in 2019 a more virulent emerging variant (emm 1 type) was described in the Lancet: Emergence of Dominant Toxigenic M1T1 Streptococcus pyogenes Clone in England.
Two weeks ago, in EID Journal: Increase of Severe Pulmonary Infections in Adults Caused by M1UK Streptococcus pyogenes, Central Scotland, UK, we looked at the near complete dominance the highly aggressive M1UK lineage of S. pyogenes in Scotland this past winter, and its apparent link to influenza A co-infection.
This week's Eurosurveillance has a lengthy and detailed report from Denmark's SSI (Statens Serum Institut) and other researchers (primarily from Iceland) on the impact and genomic developments from emerging lineages of IGAS in 2023 (including an emerging M1DK variant).While centered in the UK, increases in scarlet fever, and IGAS infections, have been reported across Europe (see last January's Denmark: SSI Reports Sharp Rise In Strep A Infections) and even in the United States (see Dec 2022's CDC HAN #0484: Increase in Pediatric Invasive Group A Streptococcal Infections).
Due to its length, and technical nature, I've only posted the link and some excerpts below. As you'll see, it isn't clear what is behind the rapid spread of the M1DK variant. If it is due primarily to societal factors (i.e. the end of COVID lockdown), then this surge would be expected to be temporary.
If, on the other hand, it is propelled more by genetic changes (i.e. more virulent or transmissible variants), then this could be the beginning of an extended uptick in infections.
Increase in invasive group A streptococcal infections and emergence of novel, rapidly expanding sub-lineage of the virulent Streptococcus pyogenes M1 clone, Denmark, 2023Thor Bech Johannesen1 , Charlotte Munkstrup2 , Sofie Marie Edslev1 , Sharmin Baig1 , Stine Nielsen2 , Tjede Funk2 , Dennis Karsten Kristensen3 , Lars Hervig Jacobsen3 , Signe Fischer Ravn3 , Niels Bindslev3 , Sophie Gubbels3 , Marianne Voldstedlund3 , Pikka Jokelainen4 , Søren Hallstrøm1 , Astrid Rasmussen1 , Karl Gústaf Kristinsson5,6 , David Fuglsang-Damgaard7 , Ram B Dessau8,9 , Agnieszka Barbara Olsén10 , Christian Salgaard Jensen11 , Annette Skovby12 , Svend Ellermann-Eriksen13 , Thøger Gorm Jensen14 , Esad Dzajic15 , Claus Østergaard16 , Steen Lomborg Andersen17 , Steen Hoffmann1 , Peter Henrik Andersen2 , Marc Stegger1,18
An increase in incidence rates of invasive (iGAS) and non-invasive (nGAS) group A Streptococcus infection has been reported by several countries across Europe during the 2022/23 winter season [1-3]. Through analysis of all whole genome sequencing (WGS) data acquired for national surveillance of iGAS in Denmark since 2018, we aimed to investigate current genomic developments and the impact of emerging lineages on iGAS incidence rates in 2023. In Denmark, iGAS is not notifiable except in case of meningitis, however, test results from all 10 Departments of Clinical Microbiology (DCMs) are submitted to the Danish Microbiology Database (MiBa) [4] and can be used to monitor incidence rates. Iceland also experienced a higher iGAS incidence in early 2023, and we also present Icelandic WGS data on iGAS isolates from 2022 and 2023.
Incidence of invasive group A Streptococcus in Denmark in 2023
We extracted all culture-positive test results for group A Streptococcus (GAS) since 2018 on 1 June 2023, and categorised them as iGAS, if the sample was from blood, synovial fluid, spinal fluid, peritoneal fluid, pleural fluid or usually sterile organ tissue, and as nGAS if the isolate was from any other sample type or from usually non-sterile organ tissue. Multiple positive test results from the same individual within a 30-day period were considered a single case. In total, we identified 1,265 cases of iGAS during the period January 2018 to May 2023 across Denmark (2023 population 5.9 million [5]).
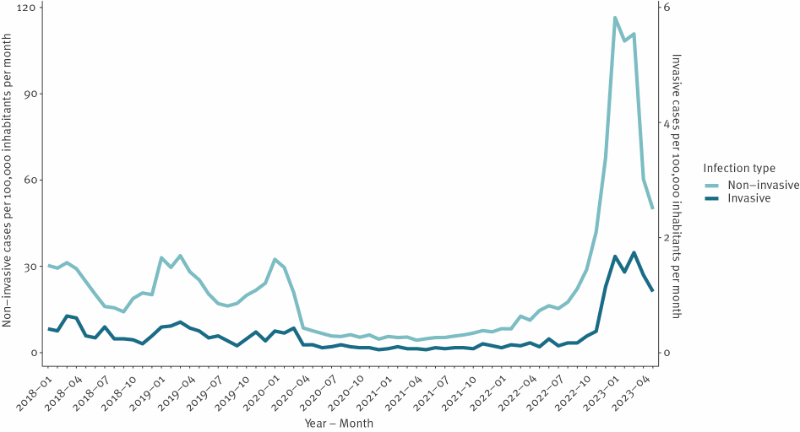
Denmark experienced historically low iGAS incidence rates during COVID-19-related restrictions, which ended in February 2022. Case numbers began to increase rapidly in November 2022, peaking in January 2023 with a monthly GAS incidence of 118 per 100,000 inhabitants, i.e. 3.5 times the peak rates seen in 2018/19, and a monthly iGAS incidence rate of 1.7 per 100,000, thus 3 times the peak rates seen in 2018/19 (Figure 1).
People 85 years or older had the highest iGAS incidence rates, peaking at 7.4 per 100,000 in the age group per month, but the highest relative increase compared with pre-COVID-19 restrictions was observed among children younger than 5 years, which peaked at 3.2 per 100,000 in the age group in March 2023. Mortality rates were similar to previous years across all age groups: 30% among people 85 years or older and less than 5% among children under 5 years. There was no substantial difference in prevalence between sexes during the winter season, with females accounting for 47.5% (222/467) of the overall cases, 41.7% (15/36) among children younger than 5 years, 52.8% (105/199) in ages 5-64 and 44.0% (102/232) among people aged 65 years and older.
(SNIP)
Discussion
Previously, the emergence of novel variants with increased capacity for virulence has been an important factor behind iGAS incidence rates. Even single nucleotide polymorphisms or indels can significantly alter iGAS virulence [7,8,11], underlining the importance of continuous surveillance of genomic trends and identification of emerging variants [12]. A shift in distribution towards the more virulent M1 variants, and the rapid spread of the M1DK lineage are both likely contributors to the high iGAS incidence in Denmark in 2023, however, these developments cannot adequately explain the surge in iGAS cases. Rather, we consider the high incidence rate of iGAS to be attributable primarily to extensive community spread. Low exposure to GAS in recent years has probably resulted in a lower level of immunity to GAS on a population level, particularly among the youngest children, a large fraction of whom had never been exposed to GAS.
It remains unclear whether the rapid expansion of M1DK is driven by an inherent competitive advantage over other variants circulating in Denmark, but we consider that the expansion is primarily enabled by the unique circumstances surrounding the implementation and subsequent lifting of COVID-19-related restrictions. Lower immunity and reduced GAS transmission during lockdowns may have enhanced the rapid expansion of individual lineages. It is, however, noteworthy that M1DK and ST36, the two variants with the largest increase in prevalence in 2022/23 compared with 2018/19, both carry the speC-gene which has been shown to facilitate nasopharyngeal colonisation in mouse models [13]. Status reports and genomic data from other countries with increased iGAS incidence, including the Netherlands [14], the UK [1,15] and Iceland (data from this study), suggest that the M1UK lineage is the leading cause of iGAS in some European countries, providing further indication that the drastic increase in cases is also driven by factors beyond genomic developments.
Conclusion
A thorough assessment of whether the surge in iGAS over winter 2022/23 was primarily driven by lowered immunity following COVID-19 related restrictions – a likely temporary circumstance, or by a higher prevalence of more virulent variants – a likely permanent change, is crucial to evaluate the risk of encountering another global increase of iGAS in coming years, and should ideally rely on whole genome sequencing data from across Europe.