Credit CDC NOWCAST As of Dec 9th
#17,827
Although its been on our radar since September, in recent weeks the JN.1 COVID variant has made huge gains - both in Europe and North America (see the CDC's Nowcast above) - debuting in second place less than 2 weeks ago.
This offshoot of the BA.2.86 variant - which carries an additional L455S mutation which likely enhances immune escape - is expected to top the charts in the next update (due Friday).
According to the CDC Update On SARS-CoV-2 Variant JN.1 published on December 9th:
- JN.1 was first detected in the United States in September 2023. By the end of October, it made up less than 0.1% of SARS-CoV-2 viruses.
- CDC projects that the variant JN.1 comprises an estimated 15–29% of in the United States as of December 8, 2023.
- CDC projects that JN.1 will continue to increase as a proportion of SARS-CoV-2 genomic sequences. It is currently the fastest-growing variant in the United States.
- Updated COVID-19 vaccines are expected to increase protection against JN.1, as they do for other variants.
- At this time, there is no evidence that JN.1 presents an increased risk to public health relative to other currently circulating variants.There is no indication of increased severity from JN.1 at this time.
Eight days ago, in Sato Lab Preprint: Virological characteristics of the SARS-CoV-2 JN.1 variant, we looked at an analysis that found that JN.1 appears to be even more immune evasive than its parental BA.2.86, which suggests that it - and its descendants - may have a growth advantage over the the older XBB Omicron lineage in the months ahead
While we've seen some disagreement over the amount of protection conferred by the new XBB COVID vaccine, it is likely that the updated vaccine provides some additional protection against infection, and it likely reduces the severity of breakthrough infections.
- COVID-19 vaccination: Vaccination coverage for the updated 2023-2024 COVID-19 vaccine remains low. As of December 2, 2023, the percent of the population reporting receipt of this vaccine was 7.7% in children 6 months–17 years (including 2.8% in children 6 months–4 years), 17.2% in adults ≥18 years (including 36% in adults ≥65 years), and 9.6% in pregnant persons.
But I'm apparently in a minority.
NEW: COVID19 variant of interest JN.1
Note: Updated with additional information for health workers and facilities
Geneva, 19 December 2023 -- Due to its rapidly increasing spread, WHO is classifying the variant JN.1 as a separate variant of interest (VOI) from the parent lineage BA.2.86. It was previously classified as VOI as part of BA.2.86 sublineages.
Based on the available evidence, the additional global public health risk posed by JN.1 is currently evaluated as low. Despite this, with the onset of winter in the Northern Hemisphere, JN.1 could increase the burden of respiratory infections in many countries.
LINK: Read the risk evaluation: https://www.who.int/activities/tracking-SARS-CoV-2-variants
WHO is continuously monitoring the evidence and will update the JN.1 risk evaluation as needed.
Current vaccines continue to protect against severe disease and death from JN.1 and other circulating variants of SARS-CoV-2, the virus that causes COVID-19.
COVID-19 is not the only respiratory disease circulating. Influenza, RSV and common childhood pneumonia are on the rise.
WHO advises people to take measures to prevent infections and severe disease using all available tools. These include:
-Wear a mask when in crowded, enclosed, or poorly ventilated areas, and keep a safe distance from others, as feasible
-Improve ventilation
-Practise respiratory etiquette - covering coughs and sneezes
-Clean your hands regularly
-Stay up to date with vaccinations against COVID-19 and influenza, especially if you are at high risk for severe disease
-Stay home if you are sick
-Get tested if you have symptoms, or if you might have been exposed to someone with COVID-19 or influenza
For health workers and health facilities, WHO advises:
-Universal masking in health facilities, as well as appropriate masking, respirators and other PPE for health workers caring for suspected and confirmed COVID-19 patients.
-Improve ventilation in health facilities
The WHO also released a more detailed risk assessment (links and excerpts follow).
Executive Summary
Previously, JN.1 was tracked as part of BA.2.86, the parent lineage that is classified as a variant of interest (VOI). However, in recent weeks, JN.1 continues to be reported in multiple countries, and its prevalence has been rapidly increasing globally and now represents the vast majority of BA.2.86 descendent lineages reported to GISAID.
Due to its rapidly increasing spread, WHO is classifying JN.1 as a separate variant of interest (VOI) from the parent lineage BA.2.86. Considering the available, yet limited evidence, the additional public health risk posed by JN.1 is currently evaluated as low at the global level. It is anticipated that this variant may cause an increase in SARS-CoV-2 cases amid surge of infections of other viral and bacterial infections, especially in countries entering the winter season.
Following discussions with the WHO Technical Advisory Group for Virus Evolution (TAG-VE) and considering the data at hand, current population immunity globally as well as immunity generated by XBB.1.5 booster vaccination is expected to remain cross-reactive to this variant, against symptomatic and severe disease. Therefore, the spread of this variant will unlikely increase the burden on national public health systems compared to other Omicron sublineages.
However, countries approaching the winter season should be aware that, altogether, SARS-CoV-2 and co-circulating pathogens may exacerbate the respiratory disease burden.
Initial Risk Evaluation of JN.1, 19 December 2023
JN.1 is a descendent lineage of BA.2.86, with the earliest sample collected on 25 August 2023 (1). In comparison with the parent lineage BA.2.86, JN.1 has the additional L455S mutation in the spike protein.
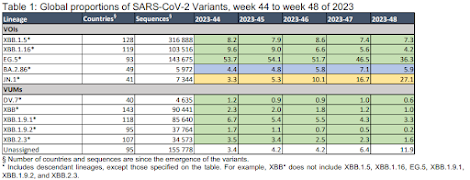
As of 16 December 2023, there were 7344 JN.1 sequences submitted to GISAID (1) from 41 countries, representing 27.1% of the globally available sequences in epidemiological week 48 (27 November to 3 December 2023). The countries reporting the largest proportion of JN.1 sequences are France (20.1%, 1552 sequences), the United States of America (14.2%, 1072 sequences), Singapore (12.4%, 934 sequences), Canada (6.8%, 512 sequences), the United Kingdom (5.6% 422 sequences), and Sweden (5.0%, 381 sequences).
Globally, there has been a rapid increase in the proportion of JN.1 reported, with its global prevalence at 27.1% in epidemiological week 48, Table 1. This is a substantial rise from the data reported four weeks prior (week 44, 30 October to 5 November 2023), when the global prevalence of JN.1 was 3.3%. This rapid growth is observed across all the three WHO regions with consistent sharing of SARS-CoV-2 sequences, i.e. the region of the Americas (AMR), the Western Pacific (WPR) and the European (EUR) regions, with the largest increase seen in WPR from 1.1% in epidemiological week 44 to 65.6% in epidemiological week 48. BA.2.86.1 (JN.1’s parent lineage) replication kinetics on primary nasal epithelial cells (hNEC) have been observed to not be higher than other XBB-derived variants (2). However, it remains to be determined whether the high transmissibility of JN.1 in humans is also associated with enhanced fitness in primary hNECs and other cell types, and how much of that is linked to non-spike mutations.
Due to differential vaccine coverage and circulation of SARS-CoV-2 variants around the world, population immunity remains heterogenous globally and therefore, the immune escape potential of JN.1 depends on the immune background of the population tested.
Whereas the immune escape of BA.2.86.1 (JN.1’s parent lineage) from XBB.1.5 and EG.5.1, breakthrough infection appears to be similar to concurrently circulating variants such as HK.3, JN.1 displays a higher immune evasion property (2,3). However, there are limited data on cross neutralization of JN.1 and despite the reduction in JN.1 neutralization, protection by XBB.1.5 monovalent vaccines are likely to be effective against JN.1 (4). WHO technical advisory groups, with scientists from around the world, are actively monitoring this (5). WHO and its Technical Advisory Group on SARS-CoV-2 Evolution (TAG-VE) continue to recommend that Member States prioritize specific actions to better address uncertainties relating to antibody escape and severity of BA.2.86 and JN.1. The suggested timelines are estimates and will vary from one country to another based on national capacities:
• Conduct neutralization assays using human sera, representative of the affected community(ies), and JN.1 live virus isolates (two to four weeks).
• Perform a comparative evaluation to detect changes in rolling or ad hoc indicators of severity (four to 12 weeks).
WHO and its Technical Advisory Group on COVID-19 Vaccine Composition (TAG-CO-VAC) continue to regularly assess the impact of variants on the performance of COVID-19 vaccines to inform decisions on updates to vaccine composition (6). The risk evaluation below follows the WHO framework (7) and is based on currently available evidence. It will be revised regularly as more evidence and data from additional countries become available.
Just over two years ago a new, milder - but more transmissible - Omicron variant burst onto the scene and quickly supplanted the Delta variant. Since then we've seen a rapid succession of Omicron variants, starting with the BA lineages and then moving on to the recombinant XBBs.
But now there is new contender (JN.1).
How long it will reign, and where it - and its descendants - will take us in 2024 and beyond is anyone's guess. But it reminds us that viral evolution doesn't stop just because we are tired of dealing with the COVID threat.