#18,097
HPAI H5N1 emerged in China nearly 30 years ago, spilling over into humans for the first time in Hong Kong in 1997. Since then it has evolved into dozens of clades, subclades, and subtypes, and while most of its early permutations are long extinct, over time the virus has broadened both its range and its diversity.
After it was successfully eradicated in Hong Kong, it disappeared for several years, only to re-emerge in Southeast Asia in 2002. Three years later it finally crossed Asia into Europe, the Middle East, and Western Africa.Its progress was slow at first.
It would take another decade before it reached North America.
Along the way the dominant subtype would shift from H5N1 to H5N8, then briefly to H5N6, and finally back to H5N1 in 2020. Since then, we've seen it greatly increase its ability to infect mammals, as well as many previously unaffected avian species.
Today the world is dealing with multiple clades and subtypes of HPAI H5, and within each are dozens of genotypes. Some are clearly more pathogenic, more transmissible, and more problematic than others.
Referral: IJID Editorial Avian `Bird' Flu - Undue Media Panic or Genuine Pandemic Concern?)
Its recent spread to American livestock (cattle, goats, alpacas) is seen as a major escalation.
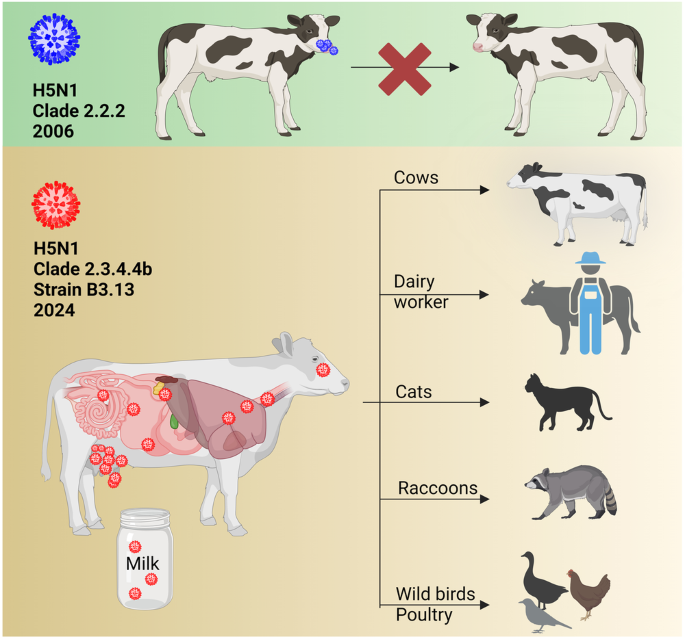
The Friedrich-Loeffler-Institut (FLI) is Germany's premier animal disease center, and they have a long history of dealing with H5N1. They've dealt with repeated epizootics of HPAI H5 in Europe (see here, here, here, here, and here), and in 2008 they successfully infected four calves with an older clade of HPAI H5N1, although they found it replicated inefficiently and produced no symptoms.
Last week they published an excellent overview/editorial on the risks of panzootic HPAIV, excerpts of which you'll find below. Martin Beer, who co-authored this paper, was one of the lead authors of the 2008 study on H5N1 in cattle.
As they point out in the following graphic, the virus's ability to spread in cattle has come a long way since 2008.
Panzootic HPAIV H5 and risks to novel mammalian hostsE. M. Abdelwhab & Martin Beer
npj Viruses volume 2, Article number: 22 (2024) Cite this article
The H5 subtype of highly pathogenic avian influenza viruses represents a significant challenge to animal and human health. H5 clade 2.3.4.4b viruses have experienced an unprecedented global spread, coupled with remarkable genetic plasticity for adaptation in birds and mammals. Although human infections remain very limited, the establishment in wild, marine, and farmed animals, including recently dairy cattle, is of concern. The role of mammalian hosts as intermediaries for zoonotic or even pandemic influenza A viruses should not be underestimated. In order to mitigate the zoonotic risk and be adequately prepared, it is essential to understand and monitor the dynamics of HPAIV H5 at the avian-mammal interface.
Diversity of avian influenza viruses
Avian influenza viruses (AIVs) have their reservoir in wild birds, especially waterfowl1. However, they also continue to cause losses in the poultry industry worldwide, affecting global meat and egg production, threatening wild bird biodiversity and posing zoonotic risks1. The influenza A virus (IAV) genome is segmented into eight distinct, negative-stranded, RNA gene segments. The error-prone nature of the IAV polymerase, which is responsible for viral replication, allows for constant change and adaptation1, which is highly advantageous for viral evolution.
When two different IAVs infect a host cell, gene segments can be swapped (i.e., reassortment or antigenic shift) to theoretically generate 254 novel genotypes2. Moreover, stepwise mutations acquired spontaneously during viral replication, may also allow the virus to adapt to new hosts (i.e. antigenic drift). To date, AIVs have been classified into 16 hemagglutinin (HA; H1-H16) and 9 neuraminidase (NA; N1-N9) subtypes based on variation in these surface glycoproteins. The type of HA and NA define the respective subtype (e.g. H5N1) with 144 possible HxNy combinations.
(SNIP)
Currently circulating H5N1 of clade 2.3.4.4b
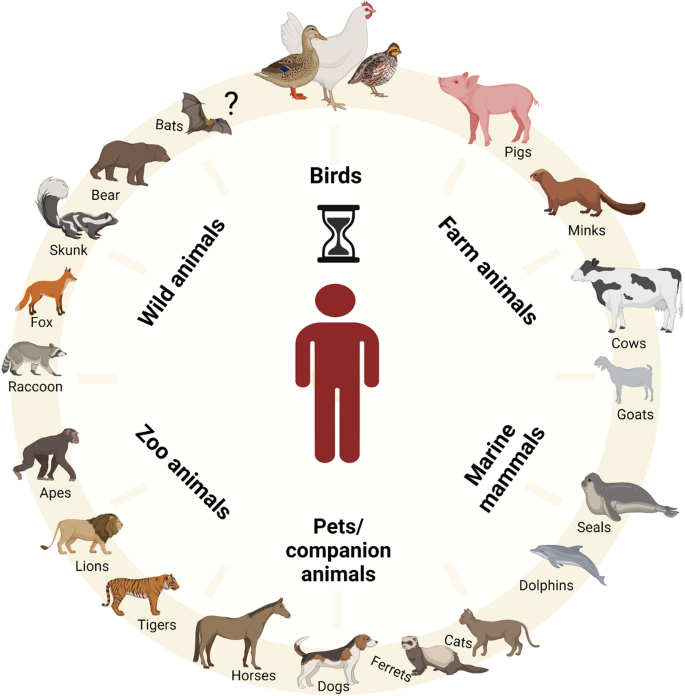
Since 2022, HPAIVs of subtype H5 have become deeply entrenched in poultry and wild birds7,8. The subsequent panzootic clade 2.3.4.4b H5-viruses have a very wide range of avian hosts9 and show numerous spill-over infections to wild carnivores, farmed fur animals such as mink, fox and raccoon dogs, and marine mammals such as sea lions10 (Fig. 1). The unique characteristics of HPAIV H5 clade 2.3.4.4b demand a fundamental rethink of our understanding of these pathogens. Europe is now a new center for HPAIV H5 in wild birds, and reassortment with other European AIV is underway11.
HPAIV H5 has been transmitted from Europe to Africa and via Iceland to North and South America. This has resulted in high overall mortality in naïve bird populations and mammals including on mainland Antarctica12,13,14. It is of great concern, that HPAIV H5 has infected well over 150 different bird species and more than 50 mammalian species (Fig. 1)15,16,17. In addition, HPAIV H5N1 of clade 2.3.4.4b has a remarkable genetic elasticity for reassortment with other AIVs resulting in a wide range of highly diverse H5N1, H5N2, H5N3, H5N4, H5N5, H5N6 and H5N8 viruses18,19.
Fig. 1: Potential hosts for the genesis of a zoonotic avian influenza virus.
Mammals infected with the current AIV H5N1 clade 2.3.4.4b present a significant risk for the emergence of zoonotic viruses. Human infections have been documented in a dairy cow farm worker. Human-adaptation markers have been identified in multiple isolates from mammals, raising concerns about the potential role of these animals as intermediate hosts for the emergence of zoonotic AIV H5N1.
HPAIV H5N1 in dairy cattle
Previous studies at the Friedrich-Loeffler-Institut, Insel Riems, Germany, showed that a 2006 H5N1 isolate from a cat replicated inefficiently in calves following intranasal high-titer infection21. Furthermore, there have been no reports of IAV circulating in cattle, although some spill-over infections of IAV have been described22.
Therefore, until recently the role of cattle in the epidemiology of IAV was considered insignificant21. In contrast, the HPAIV H5N1 clade 2.3.4.4b genotype B3.13 efficiently infected dairy cattle in 202423. Infected cattle may be subclinical or clinical and the main clinical signs are a drastic decrease in milk production, changes in milk consistency, decreased feed uptake, dehydration and fever23. The infection is reported to focus on lactating dairy cows and there are very high viral loads in the milk of infected cows. The spread seems to be mainly via the milking process and transport of infected cows to other farms in different states24.
Apparently, efficient cow-to-cow transmission has allowed the virus to spread to 63 dairy farms in at least 9 states (status as of 05/24/2024). Sequence analysis and phylodynamics suggest a single introduction(Continue . . . )several months earlier23,25. Fatal cases in cats after drinking raw milk from infected cows24 and an infection of a dairy farm worker in Texas with a conjunctivitis have been reported26. Another study described the transmission of H5N1 from dairy cows to raccoons and to wild and domestic birds27 (Fig. 2). In addition, H5N1-RNA has been detected in wastewater28, but the source of contamination remains unknown. Importantly, recent research has raised concerns that cattle, once considered a resilient host, may act as a novel IAV host in the future and after further adaptation of H5N129.
(SNIP)
Seeking answers to many questions
Influenza A viruses are masters of mutation and adaptation. There are undoubtedly serious questions to be answered about the significant change in the epidemiology of HPAIV H5 from Asia to Europe, the dynamics of infection in wild bird reservoirs, the role of climate change in the spread of the virus, the reasons for the genetic plasticity of the current clade 2.3.4.4b viruses to reassort and adapt to new hosts, and the overall low zoonotic potential of H5 viruses from this clade31,32. Vigilance is needed to prevent new waves of infection, especially in mammals, and ultimately to prevent human infection.
Dairy cows are the latest and most surprising victims in the list of AIV hosts, but they will not be the last.
Further adaptation of HPAIV H5N1 to cattle must be prevented. Stringent measures such as testing regimes (e.g. via bulk milk diagnostics), strict transport controls, quarantine measures and optimized milking hygiene must be implemented. In addition, much more basic epidemiological data is needed (e.g. seroprevalence on farms, data on non-lactating animals, H5-antibody titers in sera and milk, oro-nasal shedding data). It should also be considered that the most efficient virus shedding cows should be isolated or even euthanized. As a precautionary measure, suitable vaccines should be developed and evaluated, before the virus becomes established in these hosts.