#18,420
Just over two years ago (Apr 8th, 2023) the U.S. Fish & Wildlife Service issued a statement stating that HPAI Was Confirmed As Cause Of Death For 3 California Condors found in Northern Arizona.
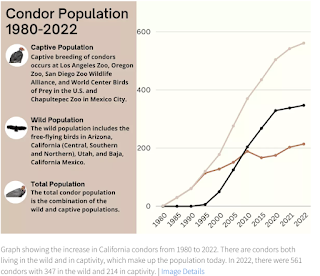
Considered a critically endangered species with a population of ≈ 560 birds today - the California Condor nearly went the way of of the Dodo in the early 1980s, when its population declined to just over 20 (in the wild) due to the combined effects of DDT, lead poisoning and a loss of habitat.This remarkable recovery was due to conservation efforts by the US Fish & Wildlife Service, the National Park Service, and breeding programs at San Diego's Wild Animal Park and the Los Angeles Zoo.
HPAI UPDATE
(Excerpt)
As of April 17, 2023, 20 condors have died in the Arizona-Utah flock; HPAI has been confirmed for 10 of those condors. Eight birds were captured and brought in for supportive care. Four of those condors died shortly thereafter and are included in the total of 20 deceased birds. Four condors are still receiving supportive care and have shown improvement. Learn more about HPAI.
In mid-May we looked at the USFWS & USDA Update On Impact of H5N1 on Condors & The Potential Use of HPAI Vaccines, which included plans to test an existing HPAI H5 poultry vaccine on vultures (a non-endangered species similar to Condors) in order to determine both its safety, and effectiveness against the currently circulating H5N1 virus (excerpt follows).
Over the summer we saw preliminary results on the vaccinated vultures, which were positive enough to allow a small test on captive condors.By October, we saw the Early HPAI Vaccine Results From Endangered California Condors, which reported that 60% of the condors (who received 2 doses) had titers that are expected to provide partial protection against mortality, while 10% of those birds had titers expected to provide protection against mortality.
On February 2nd, 2024 we saw our last substantive update from California Condor Recovery Program (excerpt below):
Based on the results of the vaccine trial, the Service determined it is appropriate to move forward with HPAI vaccinations of captively managed and free-flying condors. The Incident Command and recovery partners continue to coordinate on implementing vaccinations.
As of this report, 94 birds have received at least the initial dose of the vaccine. The vaccine is being administered with the prime and boost approach (vaccination of 0.5ml on two occasions a minimum of 21 days apart). All condors are vaccinated by veterinarians licensed in the state according to USDA/State Veterinarian-approved site-specific plans.
Antibody titers collected during the trial indicate the vaccine may reduce the severity of an infection and minimize the likelihood of mortality. Vaccinating condors with the approved vaccine may provide some protection, and even if minimal, it could reduce the amount of mortality and decrease the impact to the flock and recovery efforts if another outbreak were to occur.
Although condors did not respond to the vaccines as robustly as did black vultures, 16 (80%) of the 20 vaccinated condors seroconverted sometime during the 42-day test window, with 9 (45%) achieving HI titers >32 (thought capable of protecting against mortality) on at least 1 of the postvaccination blood draws.
While promising, there remain a lot of unanswered questions, including:
- How much real-world protection is afforded to condors from vaccination?
- How long does this protection last?
- How practical is it to capture, vaccinate, and release wild endangered birds? And how might that impact its effectiveness vs. vaccinating captive birds?
- What impact (if any) might come from viral shedding from partially protected birds?
Research
Safety and Immunogenicity of Poultry Vaccine for Protecting Critically Endangered Avian Species against Highly Pathogenic Avian Influenza Virus, United States
Todd E. Katzner , Ashleigh V. Blackford, Mary Donahue, Samantha E.J. Gibbs, Julianna Lenoch, Michael Martin, Tonie E. Rocke, J. Jeffrey Root, Darrel Styles, Sunny Cooper, Kristin Dean, Zachary Dvornicky-Raymond, Dominique Keller, Carlos Sanchez, Brett Dunlap, Thomas Grier, Michael P. Jones, Gregory Nitzel, Erin Patrick, Maureen Purcell, Aaron J. Specht, and David L. Suarez
Abstract
In 2023, an outbreak of highly pathogenic avian influenza occurred among critically endangered California condors (Gymnogyps californianus), and >21 died. We evaluated safety, immunogenicity, vaccination strategies, and correlates of antibody response of an influenza vaccine for poultry in black vultures (Coragyps atratus) and then California condors.We noted differences in antibody titers between vaccinated and unvaccinated birds (vultures p<0.004; condors p<0.02) but no adverse effects of vaccination. All vaccinated vultures and 80% of vaccinated condors showed maximum measured antibody response within the published range associated with survival of vaccinated and virally challenged chickens. We noted weak evidence of higher antibody responses for birds given two 0.5-mL vaccines versus those given one 1-mL vaccine but no correlation between antibody titers and sex for either species or between antibody titers and bone lead concentrations in vultures. Our results prompted initiation of a vaccination program for condors that could reduce spread of this disease among highly threatened species.
Discussion
Many types of HPAIV vaccines have been developed (26), including inactivated whole virus vaccines, subunit vaccines, and live vectored viral vaccines (27). Risk analysis for this vaccination trial included consideration of the potential to stimulate a protective immune response, legal availability of the vaccine, and the antigenic relatedness of vaccines to a potential field challenge. That approach led us to select the inactivated adjuvanted reverse genetics vaccine for this trial because it safely stimulated immune responses in multiple avian species and because it has ≈95.6% amino acid similarity to the currently circulating H5N1 2.3.4.4b virus isolates.
Although nearly all birds of both species responded immunologically to the vaccine, the generally stronger short-term antibody responses of black vultures compared with California condors are notable. Interspecific differences are not surprising because vaccines developed for one species can have unexpected effects in other species and related species can have substantially different responses to vaccination (28). Despite those differences, maximum HI titers of vultures given a 2-vaccine regimen were similar to those reported for domestic fowl given a similar vaccination regimen (i.e., vulture maximum titers were 32–512, chicken titers at 42 dpv were 16–1,024) (18). Similarly, maximum titers of condors given a 2-vaccine regimen were 8–64, and all but 1 titer was within the lower end of the range reported for chickens.
Work with endangered species presents many hurdles and often precludes the possibility of testing the effectiveness of a vaccine with a viral challenge (29). Furthermore, because of the dramatic impacts of HPAI on wild condors, we conducted the trials rapidly and in an extremely urgent context. Together with biosafety considerations, those issues made it impractical to conduct a logistically difficult viral challenge for the vultures or to evaluate longer-term immune response. However, we can draw inference from prior work with this vaccine. As we noted, 100% of chickens given this same vaccine responded with similar antibody levels and survived a viral challenge at 42 dpv (18). Given the similarity of those responses, had a viral challenge at 42 dpv had been feasible, we reason that most if not all vultures, and perhaps condors, would likely have survived.
The vaccine we evaluated was developed for prime-boost (2-vaccine) application. However, because trapping and handling can stress condors, we evaluated a 1-vaccine regimen as an alternative to the originally designed regimen. Despite the lack of a statistical difference between antibody responses associated with the 2 regimens, qualitative evaluation suggested that antibody responses were weaker and dissipated more rapidly for the birds that received the 1-vaccine regimen. Thus, we suspect that if birds were given a viral challenge, birds that received prime and boost vaccinations would have been more effectively protected than those vaccinated once. However, because our study ended at 42 dpv, we could not evaluate the potential for differences in waning immunity (30) between the 2 vaccine strategies.
Our failure to detect a statistical effect of either sex or bone lead concentration on antibody response might have been because of the small sample sizes and skewed sex ratios in our trials. Trends in the data suggested that if our sample size had been larger and the sex ratios more even, we might have detected differences in responses between sexes. Likewise, in the case of the response to bone lead concentrations, the statistical approach we used might not have uncovered difficult-to-detect patterns. We noted that, regardless of vaccine regimen, the birds with the strongest antibody response were also those with the lowest bone lead level. Therefore, subsequent trials might evaluate the presence of threshold-type effects in those responses.
Given the virulence and spread of the currently circulating HPAIV, vaccination of threatened and endangered wild birds could be a potential tool to mitigate losses from this disease. Vaccination may be particularly relevant when the resiliency of populations has decreased to the extent that naturally occurring illness and death from disease could impair the species’ long-term persistence. Despite the potential value of that approach, negative consequences of vaccination are possible, and this trial and implementation in the condor program are unique within the United States.Given the importance of economic considerations associated with poultry farming, close coordination with the USDA and many other federal and state agencies was essential to receive authorization to implement these vaccination trials. However, the outcomes from these trials were positive enough, and the threat from HPAIV so great, that the USFWS subsequently decided to initiate a vaccination program for the California Condor Recovery Program (11). By October 2024, a total of 207 condors had received >1 vaccination (30).
Species-specific variations in physiological response to vaccination are characteristic problems associated with vaccination programs for wildlife (31). Despite such variations, evidence suggests that vaccination strategies that reach <50% of an affected wildlife population can still be effective at staving off extinction (32,33). Those trends, together with results of our work, suggest several next steps for protecting endangered wildlife, whether condors or other species, from infectious diseases. Vaccination of the at-risk population of the target species can begin once safety, immunogenicity, and vaccination regimens have been established and some correlate of protection established, either from published work with other species or from direct challenge trials.Critical next steps include monitoring vaccine effectiveness in field settings and demographic modeling to understand the most effective strategy for vaccination of wild animals. Specifically, given the challenges inherent in vaccinating wild animals, using life history traits of the species in question, together with population modeling, can confirm the costs and benefits of vaccination in relation to its risks and, thus, can help identify vaccination strategies that can stabilize populations and enable them to recover. Relevant vaccination strategies might involve varying the time of year (especially relative to reproductive seasons and seasonal variation in survivorship), age classes, and proportion of the population that is vaccinated.
In summary, we evaluated safety, immunogenicity, vaccination regimens, and correlates of antibody response for a conditionally licensed influenza subtype H5N1 vaccine designed for poultry in black vultures and California condors. Our work suggests that the use of licensed vaccines can be a realistic strategy to aid in conservation of condors and potentially other species facing similar threats, especially those with small and highly threatened populations.
Dr. Katzner is a research wildlife biologist at the USGS, Forest and Rangeland Ecosystem Science Center in Boise, Idaho, USA. His research interests focus on birds of prey, renewable energy, and conservation biology.
The stark difference in HI titers >32 between vultures (90%) and condors (60%) receiving two doses of the vaccine is a reminder that one size rarely fits all, and that there is more work to be done on refining H5 vaccines.
Given that HPAI isn't likely to go away anytime soon, creating more effective vaccines that are protective across a wider range of species should remain a priority.