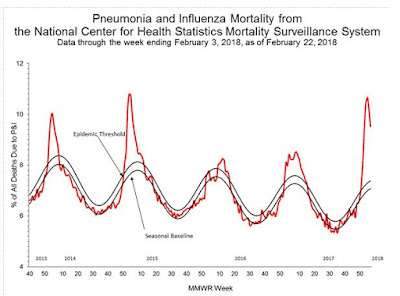
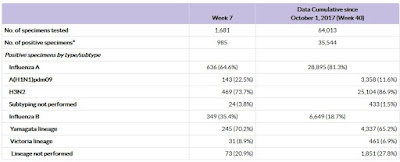
#13,170
Like flu and hurricane seasons, our spring tornado and severe storm season varies in intensity every year, but even in a mild year hundreds of tornadoes will be spawned across the Midwest and Deep South over the spring and early summer.
Today, the NOAA/NWS Storm Prediction Center has issued a forecast calling for an enhanced risk of severe thunderstorms across a wide swath running from Northeastern Texas to the Ohio Valley.
While we've seen a string of mild-to-moderate tornado seasons over the past 6 years, in
2011 - during a three days (
Apr 25th-28th) - a storm system of epic proportions generated 351 confirmed tornadoes across five southern states, killing
338 persons
in Alabama, Arkansas, Georgia, Mississippi, and Tennessee.
This was
the the third deadliest tornado outbreak in U.S. History. More than a
dozen of these twisters reached intensities of 4 or 5 on the Enhanced
Fujita [
EF] scale, which can produce near total devastation.
All but a small part of the United States is vulnerable to these storms, and while more common in the spring and summer, they can happen anytime of the year.
The strongest generally occur in an area we call
Tornado Alley
(
below Left), which runs from middle Texas north though Oklahoma,
Kansas, Nebraska and South Dakota. This is the area where you will
generally find the largest and most powerful tornadoes; the F5 wedge
type.
 |
| Tornado Alley - Dixie Alley |
Fortunately, much of the mid-west is sparsely populated, and so the
number of tornado deaths that occur here are actually less than in other
areas of the country.
DIXIE ALLEY (above right) sees more frequent, albeit usually less
severe tornadoes. Due to a higher population density, more deaths
occur in Dixie Alley than in Tornado Alley most years.
Which is why every home and office should have a
NOAA weather radio.
Once thought of as mainly a source of local weather information, it has
now become an `All-Hazards' alert system as well.
Having a safe place to go in your home during a tornado can be life saving. A basement is best, but an interior hallway or windowless room may provide some protection as well.
In 2012 the
CDC’s MMWR issued an analysis of the 2011 massive tornado outbreak, that stressed the importance of
safe rooms. Due to the length of the report, I’ve only reproduced a few excerpts.
Follow the link to read:
Tornado-Related Fatalities — Five States, Southeastern United States, April 25–28, 2011
July 20, 2012 / 61(28);529-533
(Media Synopsis)
Individuals who work or live in a tornado-prone area should develop a tornado safety plan prior to severe weather.
During
April 25–28, 2011, the third deadliest tornado disaster occurred in the
southeastern U.S. despite modern advances in tornado forecasting,
advanced warning times, and media coverage. CDC reviewed data from the
American Red Cross, death certificates and the National Weather Service
to describe the fatalities by demographic characteristics, shelter used,
cause of death, and tornado severity in the affected states of Alabama,
Arkansas, Georgia, Mississippi and Tennessee. Of the 338 deaths,
approximately one-third were older adults, almost half occurred in
single-family homes, and a quarter happened in mobile homes. One-half
of the 27 tornadoes were rated powerful (EF-4 or EF-5) and were
responsible for almost 90 percent of the deaths. The use of safe rooms is crucial to preventing tornado-related deaths.
(Continue . . . .)
FEMA has a good deal of advice on exactly how to construct a safe room – either above or below ground.
Residential Safe Rooms
The
information below will help you understand how having a safe room in
your home can protect your family and save the lives of those you care
about.
Find answers to your Questions about Building a Safe Room, including:
- What is the cost of installing a safe room?
- Can I install a safe room in an existing home?
- Can I build the safe room myself?
- Where is the best location for the safe room?
- Where can I find plans for safe room construction?
And more....
Building a Safe Room in Your House
For more details about how you can build a safe room in your home, go to the FEMA P- 320, Taking Shelter from the Storm: Building a Safe Room for Your Home or Small Business page before downloading it from the FEMA Library.
Having a good (
and well rehearsed) family emergency plan is essential for any disaster. Even with a safe room, family members could become separated
(they may be sent to different hospitals or shelters) in the post-disaster chaos.
Some may be injured and unable to provide information about their families.
So it is important to set up a plan, including meeting places and out-of-state contacts, and
individual wallet information cards - before you need it (see
#NatlPrep : Create A Family Communications Plan).
Together with
adequate emergency supplies, a solid
first aid kit, and an emergency
battery operated NWS Weather Radio, these steps will go a long ways to protecting you, and your family, from a wide variety of potential disasters.
For more on all of this, a partial list of some of my preparedness blogs include:
When 72 Hours Isn’t Enough
In An Emergency, Who Has Your Back?
#NatlPrep: The Gift Of Preparedness 2017