#16,563
If there is one overriding takeaway from writing this blog for 16+ years, it's that nature's laboratory is open 24/7 and it never stops tweaking its biological experiments. We've seen it time and time again. A formerly obscure virus suddenly gains new abilities, and then begins to impact new species.
COVID is the most recent example, but the past 25 years is filled with others, including:
- H5N1, which emerged in Hong Kong, infecting 18 people in 1997. Since then it has diverged into numerous avian flu subtypes (H5N1, H5N8, H5N6, etc.) threatening poultry hand humans.
- Nipah, which emerged in 1998 in Malaysia - which spread from bat to pigs, and then from pigs to humans - eventually infecting at least 265 people, killing 105 (see Lessons from the Nipah virus outbreak in Malaysia).
- SARS-CoV, which emerged in 2002, and sparked a global epidemic.
- MERS-CoV, which emerged in 2012, which has infected more than 2,500 people killing 35%.
- H7N9, a low path virus that emerged in China in 2013, and while harmless to chickens, was deadly to humans.
- Zika and Chikungunya, both obscure mosquito-borne viruses native to Africa, which mutated over the past 15 years to become more transmissible, and they went on a world tour.
- SARS-CoV-2, our current pandemic, that until December of 2019 was virtually unknown.
This is but a short list, but is shows that evolution never stops. The virus, bacteria, or fungus we peacefully coexist with today could become an formidable threat tomorrow.
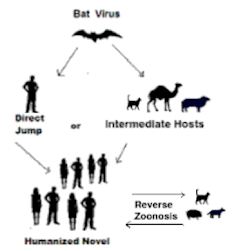
And most of the emerging infections diseases we've seen over the past 50 years have been zoonotic; arising from a variety of non-human animal host (bats, birds, pigs, etc.).
The ability for potential zoonotic diseases to evolve in non-human hosts makes the spread of COVID to other species so much of a concern.
In November of 2020, we saw several new strains of COVID emerge from infected farm-raised mink, and go on to infect humans in the community (see Denmark Orders Culling Of All Mink Following Discovery Of Mutated Coronavirus).
While supplanted by a more aggressive Alpha variant, this accidental field experiment proved a point. Humans could infect other species with COVID, and those new hosts could generate a new variant, which could jump back into humans.
This history with mink helps to explain Hong Kong's robust response when hamsters in local pet shops tested positive for the virus last month (see Hong Kong: Compulsory Quarantine For Those Exposed To COVID-Positive Hamsters).
Fortunately, most farmed animals (pigs, chickens, cattle, etc.) are poor hosts for the SARS-CoV-2 virus. Dogs and cats are mildly susceptible, but since they don't have contact with hundreds of other animals, aren't as likely to generate mutations.
While there are several species in the wild that are believed capable of hosting, and spreading, SARS-CoV-2,so far North American Deer are showing the greatest promise.
We've revisited this topic several times, including COVID Reservoir Roundup: Cambodian Bats, Farmed Mink In Utah & North American Deer and Two New Reports Find Widespread SARS-CoV-2 In North American Deer).
Over the past few days, a couple of new studies have been released on COVID and North American Deer, including the following preprint (not yet peer-reviewed) report on the Omicron variant in deer on Staten Island, New York.
Detection of SARS-CoV-2 Omicron variant (B.1.1.529) infection of white-tailed deer
Kurt J. Vandegrift, Michele Yon, Meera Surendran-Nair, Abhinay Gontu, Saranya Amirthalingam, Ruth H. Nissly, Nicole Levine, Tod Stuber, Anthony J. DeNicola, Jason R. Boulanger, Nathan Kotschwar, Sarah Grimké Aucoin, Richard Simon, Katrina Toal, Randall J. Olsen, James J. Davis, Dashzeveg Bold, Natasha N. Gaudreault, Juergen A. Richt, James M. Musser, Peter J. Hudson, View Vivek Kapur, Suresh V. Kuchipudi
doi: https://doi.org/10.1101/2022.02.04.479189
Abstract
White-tailed deer (Odocoileus virginianus) are highly susceptible to infection by SARS-CoV-2, with multiple reports of widespread spillover of virus from humans to free-living deer. While the recently emerged SARS-CoV-2 B.1.1.529 Omicron variant of concern (VoC) has been shown to be notably more transmissible amongst humans, its ability to cause infection and spillover to non-human animals remains a challenge of concern.We found that 19 of the 131 (14.5%; 95% CI: 0.10–0.22) white-tailed deer opportunistically sampled on Staten Island, New York, between December 12, 2021, and January 31, 2022, were positive for SARS-CoV-2 specific serum antibodies using a surrogate virus neutralization assay, indicating prior exposure.The results also revealed strong evidence of age-dependence in antibody prevalence. A significantly (χ2, p < 0.001) greater proportion of yearling deer possessed neutralizing antibodies as compared with fawns (OR=12.7; 95% CI 4–37.5). Importantly, SARS-CoV-2 nucleic acid was detected in nasal swabs from seven of 68 (10.29%; 95% CI: 0.0–0.20) of the sampled deer, and whole-genome sequencing identified the SARS-CoV-2 Omicron VoC (B.1.1.529) is circulating amongst the white-tailed deer on Staten Island.
Phylogenetic analyses revealed the deer Omicron sequences clustered closely with other, recently reported Omicron sequences recovered from infected humans in New York City and elsewhere, consistent with human to deer spillover. Interestingly, one individual deer was positive for viral RNA and had a high level of neutralizing antibodies, suggesting either rapid serological conversion during an ongoing infection or a “breakthrough” infection in a previously exposed animal.Together, our findings show that the SARS-CoV-2 B.1.1.529 Omicron VoC can infect white-tailed deer and highlights an urgent need for comprehensive surveillance of susceptible animal species to identify ecological transmission networks and better assess the potential risks of spillback to humans.
Key FindingsThese studies provide strong evidence of infection of free-living white-tailed deer with the SARS-CoV-2 B.1.1.529 Omicron variant of concern on Staten Island, New York, and highlight an urgent need for investigations on human-to-animal-to-human spillovers/spillbacks as well as on better defining the expanding host-range of SARS-CoV-2 in non-human animals and the environment.
Another study, this time published in PNAS, finds:
Multiple spillovers from humans and onward transmission of SARS-CoV-2 in white-tailed deer
Suresh V. Kuchipudi, Meera Surendran-Nair, Rachel M. Ruden, Michele Yon, Ruth H. Nissly, Kurt J. Vandegrift, Rahul K. Nelli, Lingling Li, Bhushan M. Jayarao, Costas D. Maranas, Nicole Levine, Katriina Willgert, Andrew J. K. Conlan, Randall J. Olsen, James J. Davis, James M. Musser, View Peter J. Hudson, and Vivek Kapur
See all authors and affiliations
PNAS February 8, 2022 119 (6) e2121644119; https://doi.org/10.1073/pnas.2121644119
Significance
The results provide strong evidence of extensive SARS-CoV-2 infection of white-tailed deer, a free-living wild animal species with widespread distribution across North, Central, and South America. The analysis shows infection of deer resulted from multiple spillovers from humans, followed by efficient deer-to-deer transmission. The discovery of widespread infection of white-tailed deer indicates their establishment as potential reservoir hosts for SARS-CoV-2, a finding with important implications for the ecology, long-term persistence, and evolution of the virus, including the potential for spillback to humans.
Abstract
Many animal species are susceptible to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and could act as reservoirs; however, transmission in free-living animals has not been documented. White-tailed deer, the predominant cervid in North America, are susceptible to SARS-CoV-2 infection, and experimentally infected fawns can transmit the virus.To test the hypothesis that SARS-CoV-2 is circulating in deer, 283 retropharyngeal lymph node (RPLN) samples collected from 151 free-living and 132 captive deer in Iowa from April 2020 through January of 2021 were assayed for the presence of SARS-CoV-2 RNA. Ninety-four of the 283 (33.2%) deer samples were positive for SARS-CoV-2 RNA as assessed by RT-PCR. Notably, following the November 2020 peak of human cases in Iowa, and coinciding with the onset of winter and the peak deer hunting season, SARS-CoV-2 RNA was detected in 80 of 97 (82.5%) RPLN samples collected over a 7-wk period.Whole genome sequencing of all 94 positive RPLN samples identified 12 SARS-CoV-2 lineages, with B.1.2 (n = 51; 54.5%) and B.1.311 (n = 19; 20%) accounting for ∼75% of all samples. The geographic distribution and nesting of clusters of deer and human lineages strongly suggest multiple human-to-deer transmission events followed by subsequent deer-to-deer spread.These discoveries have important implications for the long-term persistence of the SARS-CoV-2 pandemic. Our findings highlight an urgent need for a robust and proactive “One Health” approach to obtain enhanced understanding of the ecology, molecular evolution, and dissemination of SARS-CoV-2.
The overriding concern is that deer - or some other non-human species - could server as a reservoir host for COVID, and over time the virus could mutate into a new, and potentially more dangerous, variant that could then jump back into humans.
Five months ago, China's CCDC Weekly published a perspective article by two Chinese scientists that readers of this blog are already familiar with - George F. Gao and Liang Wang - on the continual spread of SARS-CoV-2 from humans to other animal hosts, and the impacts that could have going forward.
(Excerpt)
THE HOST EXPANSION OF SARS-COV-2 IS NOT OVER
The host expansion of coronaviruses was well established (15). A previous study found some ongoing mink-adapted mutations such as Y453F, F486L, and N501T in the S protein; for example, Y453F has been found to increase in hACE-2 affinity (16). Those results suggested that some ongoing mink-adapted mutations posed a huge threat to public health if they transmitted back to humans and even triggered further community transmission. Due to the fact that minks are bred in farms, large-scale slaughter of these minks can effectively prevent mink-derived SARS-CoV-2 variants from spreading and mutation accumulation in the mink population. However, similar measures could not be taken for wild animals.
Together with the fact that adaptive mutations are needed when cross-species transmission happens and then circulate among populations of the new host, more efforts are needed to survey the genetic alterations and corresponding impact of transmissibility and infectivity in humans in these novel variants from wild white-tailed deer. Since SARS-CoV-2 is going wild, many other wild animals would also be infected with SARS-CoV-2 via direct or indirect contact with wild white-tailed deer or even infected patients.
Several experimental studies have demonstrated several animals could be susceptible to SARS-CoV-2, such as Egyptian fruit bats (Rousettus aegyptiacus), marmosets (Callithrix jacchus), macaques (Macaca fascicularis and Macaca mulatta), bank voles (Myodes glareolus), and North American deer mice (Peromyscus maniculatus) (10).
However, these are just the tip of the iceberg as the susceptibility of most terrestrial wild animals to SARS-CoV-2 has not been tested. In addition, the research on susceptibility of marine wildlife (especially marine mammals) to SARS-CoV-2 is still lacking. Due to frequent marine human activities (such as mariculture and marine fishing), the frequency of human contact with marine organisms is high. If some marine organisms are highly susceptible to SAR-CoV-2, there is a risk that SARS-CoV-2 could be transmitted from humans to marine organisms, and worse, SARS-CoV-2 then might spread in the marine ecosystem, which may lead to the generation of some novel SARS-CoV-2 variants with unknown threats to humans.
Therefore, it is necessary to carry out large-scale SARS-CoV-2 screening for terrestrial and marine wildlife, especially those susceptible ones, in order to monitor the status of infection and mutation of SARS-CoV-2 in wild animals, so as to formulate further prevention and control strategies. It also provides more clues to the study of the origin and cross-species transmission of SARS-CoV-2.
Acknowledgement: Dr. Kefang Liu and Mr. Linjie Li for help in collecting the references and drawing figure.
Just as we monitor the spread and evolution of influenza viruses carried by pigs, birds, and marine mammals due to their pandemic potential, the expansion of SARS-CoV-2 in a growing number of animals hosts will likely present a similar threat to public health going forward.
Because ready or not, the next pandemic is coming.