#16,865
Although the WHO's emergency committee declined to declare the global outbreak of Monkeypox a PHEIC (Public Health Emergency of International Concern) twelve days ago, the number of confirmed cases has grown steadily, and Director Tedros has publicly announced his intention to reconvene the committee later this month.
In another sign that this outbreak has `legs', yesterday the WHO transitioned from publishing irregular updates via their DON (Disease Outbreak News) format to a more formal bi-weekly Situation Report (#1).
Due to its length, I've only posted some excerpts from the 11-page PDF below. Follow the link to read it in its entirety.
External Situation Report 1, published 6 July 2022
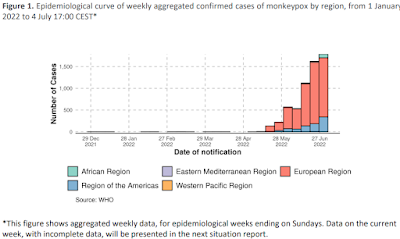
Data as received by WHO national authorities by 17:00 CEST, 4 July 2022
Highlights
• Updates on the multi-country outbreak of monkeypox has transitioned from the Disease Outbreak News to a biweekly situation report. The situation report will provide details such as the latest epidemiology, new guidance documents and updates on WHO advice. For information not included in this report, please see the Disease Outbreak News published on 27 June 2022.
• WHO published a guidance document to provide public health advice for gatherings during the current monkeypox outbreak on 28 June. The advice was developed for host governments, public health authorities, national or international organizers, and professional staff involved in the planning and delivery of gatherings, including people organizing smaller gatherings or attending gatherings of any type and size.
• The outbreak continues to primarily affect men who have sex with men who have reported recent sex with one or multiple male partners, suggesting no signal of sustained transmission beyond these networks for now.
Epidemiological Update
From 1 January to 4 July 2022, 6027 laboratory confirmed cases of monkeypox and three deaths have been reported to WHO from 59 countries/territories/areas in five WHO Regions (African Region, Region of the Americas, Eastern Mediterranean Region, European Region, Western Pacific Region) (Table 1). Since the previous Disease Outbreak News was published on 27 June 2022, 2614 new cases, (77% increase) and two new deaths have been reported; nine new countries/territories/areas have reported cases. Ten countries have not reported new cases for over 21 days, the maximum duration of the incubation period of the disease. This is the first time that local transmission of monkeypox has been reported in newly-affected countries without epidemiological links to countries that have previously reported monkeypox in West or Central Africa.
Case demographics
Data on sex are available for 73% (4406/6027) of cases. Of these, 99.5% (4385/4406) are males, and the median age of reported cases is 37 years (Interquartile range: 31-43). Males between 18-44 years of age continue to be disproportionately affected by this outbreak as they account for 79% of cases. 0.1% (6/5584) of cases with age data are aged 0-17 years of age.
Although the vast majority of cases continue to be reported from Europe, the United States has identified just over 600 cases (as of July 6th). Although it is improving, limited testing capacity by the CDC's National laboratory has been a serious obstacle.
Yesterday the CDC announced the first availability of orthopoxvirus testing by a commercial lab (Labcorp), which should double the testing capacity in the United States to 20,000 a week.
Labcorp To Begin Monkeypox Testing Today, Doubling Nationwide Testing Capacity
Media Statement
For Immediate Release: Wednesday, July 6, 2022
Contact: Media Relations
(404) 639-3286
Starting today, Labcorp will begin testing for monkeypox using CDC’s orthopoxvirus test (which detects all non-smallpox related orthopoxviruses, including monkeypox).
“The ability of commercial labs to test for monkeypox is a key pillar in our comprehensive strategy to combat this disease,” said CDC Director Rochelle Walensky, M.D., M.P.H. “This will not only increase testing capacity but will make it more convenient for providers and patients to access tests by using existing provider-to-lab relationships.”
Labcorp will offer this testing at its largest facility in the United States and will be able to accept specimens from anywhere in the country. Labcorp expects to be able to perform up to 10,000 tests per week, which will double the current capacity provided through CDC’s Laboratory Response Network (LRN), which itself has rapidly expanded testing capacity over the last seven weeks.
On June 22, HHS announced that five commercial laboratory companies would soon begin offering monkeypox testing. Since then, CDC shipped the tests to the laboratories and their employees have been trained on their administration, among other steps.
Anyone with a rash that looks like monkeypox should talk to their healthcare provider about whether or not they need to get tested, even if they don’t think they had contact with someone who has monkeypox. Healthcare providers, nationwide, can order the orthopoxvirus test from Labcorp just as they normally would order other tests. The public will not be able to go to a Labcorp lab and submit a specimen. Labcorp will use electronic laboratory reporting (ELR) to report results to jurisdictions as outlined in the CDC reporting guidance.
CDC anticipates additional commercial laboratories will come online and monkeypox testing capacity will continue to increase throughout the month of July. Healthcare providers can access information on Labcorp’s test at www.labcorp.com/monkeypox*. The latest CDC information on monkeypox is available at www.cdc.gov/monkeypox.