#17,133
Almost exactly a year ago we witnessed a seismic change in the SARS-CoV-2 virus when a new, and radically different COVID variant (B.1.1.529 variant (dubbed Omicron) emerged in South Africa, and quickly conquered the world.
While controversial, some scientists believe the Omicron variant may have evolved after the virus jumped to mice or other rodents (see Evidence for a mouse origin of the SARS-CoV-2 Omicron variant), and then spilled back into humans (see Maryn McKenna's article in Wired).
The idea isn't so far-fetched, as we've previously seen a COVID variant emerged from farmed mink in Denmark, and then spread through the population (see WHO 2nd Update: SARS-CoV-2 mink-associated variant strain – Denmark).
We've seen other cases where non-human hosts appear to have infected humans, often with a `genetically distinct' variant, including:
Divergent SARS-CoV-2 variant emerges in white-tailed deer with deer-to-human transmission
CDC: Investigating Possible Mink-To-Human Transmission Of SARS-CoV-2 In The United States
EID Journal: Suspected Cat-to-Human Transmission of SARS-CoV-2 - Thailand
The potential harm from SARS-CoV-2 establishing itself in one or more non-human host species is great enough that last March world health organizations (WHO/FAO/OIE) published a Joint Statement On Monitoring SARS-CoV-2 In Wildlife & Preventing Formation of Reservoirs.
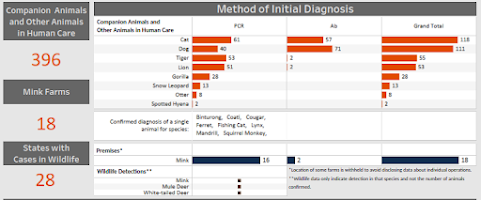
The USDA maintains a dashboard showing confirmed cases of SARS-CoV-2 in animals, although it undoubtedly only represents a small fraction of actual infections.
Not surprisingly, companion animals (cats & dogs) lead the list, but how often they are infected is unknown.
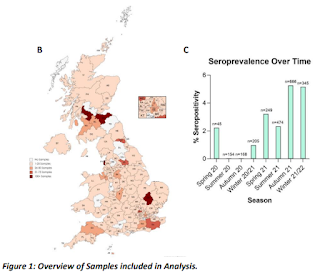
Shedding some light on this topic, we have a preprint out of the UK which - using blood samples submitted to University of Glasgow Veterinary Diagnostic Services laboratory (VDS) over nearly a two year period (Apr 2020-Feb 2022) - retrospectively finds the seroprevalence of SARS-CoV-2 in cats to average 3.2% (peaking at 5.3%).
These findings are all pre-Omicron, since that variant had only been spreading widely for a short time by the cut-off time for this study.
The authors noted:
A higher proportion of pedigree cats were seropositive compared to their non-pedigree counterparts - this finding approached statistical significance. Pedigree cats are more likely to be indoor-only (68) and may therefore experience more close contact with their owners, meaning they are more exposed to SARS-CoV-2 if their owners become infected.
The full preprint is well worth reading. I'll have a postscript when you return.
Rising SARS-CoV-2 Seroprevalence and Patterns of Cross-Variant Antibody Neutralization in UK Domestic Cats
Grace B Tyson, Sarah Jones, Nicola Logan, Michael McDonald, Pablo R Murcia, Brian J Willett, William Weir, Margaret J Hosie
doi: https://doi.org/10.1101/2022.11.18.517046
Abstract
Recent evidence confirming cat-to-human SARS-CoV-2 transmission has highlighted the importance of monitoring infection in domestic cats. Although the effects of SARS-CoV-2 infection on feline health are poorly characterized, cats have close contact with humans, and with both domesticated and wild animals.Accordingly, they could act as a reservoir of infection, an intermediate host and a source of novel variants.To investigate the spread of the virus in the cat population, serum samples were tested for SARS-CoV-2 antibodies by ELISA and a pseudotype-based virus neutralization assay, designed to detect exposure to variants known to be circulating in the human population.Overall seroprevalence was 3.2%, peaking at 5.3% in autumn 2021. Variant-specific neutralizing antibody responses were detected with titers waning over time. The variant-specific response in the feline population correlated with and trailed the variants circulating in the human population, indicating multiple ongoing human-to-cat spill-over events.
(SNIP)
Our results demonstrate the importance of widespread testing of cats, to detect SARS-CoV-2 exposure and better understand the morbidity and mortality associated with infection in cats. Testing oropharyngeal swabs for SARS-CoV-2 RNA by RT-qPCR provides an opportunity to monitor for feline specific mutations and transmission events from infected cats, as well as allowing for comparison with serology to accurately identify the causative variant of infection.
Both widespread serological and qPCR-based testing are vital to address the One Health aspect of SARS-CoV-2 infection (70). Without further research to determine the importance of cats as a possible SARS-CoV-2 reservoir, a vital piece of the jigsaw may be missing in the attempt to bring global infections under control.
After three long years of the COVID pandemic, the world's hopes are pinned on the idea that - given enough time - the SARS-CoV-2 virus will settle down into an endemic, and less impactful, seasonal respiratory virus.
While COVID has lost some of its severity, it continues to reinvent itself into new, highly transmissible variants.
The wildcard out there is the possibility that a new, highly divergent variant might emerge from a non-human host reservoir (mink, deer, mice, dogs, cats, etc.) that not only reinvigorates the pandemic, but increases the severity of the virus in humans.
How likely that is to happen is anyone's guess. But we can't afford to ignore the possibility.