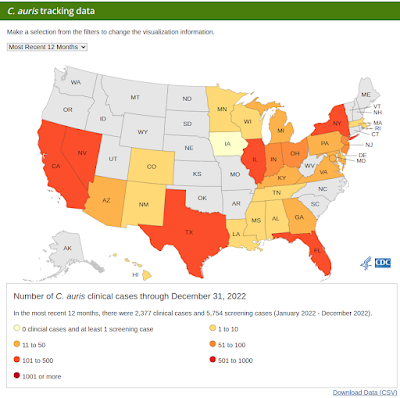
Credit CDC
#17,492
Seven years ago this month the CDC issued a Clinical Alert to U.S. Healthcare facilities about the Global Emergence of Invasive Infections Caused by the Multidrug-Resistant Yeast Candida auris, which had been detected in nine countries on four continents since 2009.
A week later we saw a report from the UK's PHE on an outbreak that had been ongoing there since 2015 (see PHE On The Emergence Of Candida auris In The UK).
Unlike most systemic Candida infections, which usually arise when a previously colonized person is weakened from illness or infirmity, this strain has a propensity for nosocomial transmission.C. auris is an emerging fungal pathogen that was first isolated in Japan in 2009. It was initially found in the discharge from a patient's external ear (hence the name `auris'). Retrospective analysis has since traced this fungal infection back over 20 years.
Additionally:
- C. auris infections have a high fatality rate
- The strain has developed resistance to multiple classes of anti-fungals
- This strain is unusually persistent on fomites in healthcare environments.
- And it can be difficult for labs to differentiate between Candida strains
We last looked at C. auris in early May in The Lancet: Candida auris - An Emerging Antimicrobial-resistant Organism with the Highest Level of Concern.
Research Letter
Candida auris‒Associated Hospitalizations, United States, 2017–2022
Kaitlin Benedict, Kaitlin Forsberg, Jeremy A.W. Gold, James Baggs, and Meghan Lyman
Author affiliation: Centers for Disease Control and Prevention, Atlanta, Georgia, USA
Abstract
Using a large US hospital database, we reviewed 192 Candida auris‒associated hospitalizations during 2017–2022, including 38 (20%) C. auris bloodstream infections. Hospitalizations involved extensive concurrent conditions and healthcare use; estimated crude mortality rate was 34%. These findings underscore the continued need for public health surveillance and C. auris containment efforts.
Candida auris is a highly transmissible and frequently drug-resistant emerging fungal pathogen capable of causing severe infections. C. auris can colonize skin, leading to infection and transmission in healthcare settings. In the United States, reported clinical cases increased by 95% during 2020–2021 (1). US data on C. auris come primarily from case series and outbreak investigations and are geographically limited, and national surveillance data lack detail on patients’ underlying conditions, healthcare use, and outcomes. Therefore, we used a large healthcare services database to describe features of hospitalized patients with C. auris infection or colonization.
(SNIP)
A total of 192 C. auris hospitalizations (38 [20%] with BSI) occurred in 42 hospitals. C. auris hospitalizations primarily occurred among older adults (median age 68 years [range 21–89 years]), male patients (54%), and non-Hispanic White patients (60%). Non-Hispanic Black patients more frequently had BSI than did other races/ethnicities (39% vs. 29%; p = 0.022) (Table). The first positive C. auris specimen was collected within 2 days of admission for 63% of bloodstream and 48% of nonbloodstream C. auris hospitalizations. Among hospitalizations with bloodstream C. auris, 58% also had another positive specimen type. Among hospitalizations without bloodstream C. auris, the most common positive specimen types were axilla (38%) and urine (34%).
Underlying conditions and complications were similar for patients with bloodstream and nonbloodstream C. auris and most commonly were sepsis (64%), diabetes (55%), chronic kidney disease (44%), and pneumonia (43%). Compared with nonbloodstream C. auris, bloodstream C. auris hospitalizations more frequently involved central venous catheters (CVC) (76% vs. 53%; p = 0.010) and tracheostomies (29% vs. 12%; p = 0.008). Echinocandin use was more frequent for bloodstream (76%) vs. nonbloodstream (25%) hospitalizations; median time from first positive culture to echinocandin use was 2 days (interquartile range 1–3 days).
Most (75.5%) hospitalizations involved an intensive care unit stay; mechanical ventilation was used in 43% of intensive care unit cases. Median hospitalization length was 13 days (range, 1–209 days). In-hospital mortality rate was 21%; discharge locations included hospice (13%), skilled nursing facility (28%), and long-term acute care (15%). Estimated crude mortality rates were 47% for bloodstream C. auris vs. 31% for nonbloodstream.
This analysis of a large convenience sample of C. auris‒associated hospitalizations provides information about clinical features that are currently unavailable through national public health surveillance. Our results support smaller previous investigations showing that infection and colonization with C. auris occurs most commonly in patients with complex medical conditions (2–5). The proportion of C. auris cases involving BSI (20%) was comparable to the 9%–28% BSI rate among clinical and screening cases found in previous state-specific studies (2,6). Including in-hospital deaths and discharges to hospice, the overall estimated crude mortality rate of 34% (47% for BSI) was similar to the 30-day mortality rate from a previous study in New York (27% overall and 39% for BSI) (2).
Consistent with candidemia treatment guidelines, most BSI hospitalizations involved echinocandin use and typical treatment lag (7). Hospitalizations involving C. auris BSI were associated with non-Hispanic Black race, similar to those for non–C. auris candidemia (8). The association between CVC use and C. auris BSI is not surprising, given that CVC use is a well-documented risk factor for candidemia and is common among patients with C. auris, because extensive healthcare exposure, intensive care unit stays, and use of medical devices are key factors in C. auris acquisition (2,9). Many BSI patients were probably admitted with C. auris, based on first positive blood specimens occurring soon after admission, sNeither clade I nor clade III had circulated in Israel before 2021, suggesting they arose through importation events into the country.imilar to finding from a New York case series (3). However, we could not assess previous healthcare exposures and prehospitalization laboratory data, which are major considerations because patients with C. auris usually acquire it in healthcare settings and can remain colonized for months (9).
Other limitations of our study include lack of antifungal susceptibility testing data, possible underdetection of cases caused by potential incompleteness of 2022 data, and underrepresentation of the West and the Northeast regions in PHD laboratory data (10), which is particularly relevant because those regions report high case counts (https://www.cdc.gov/fungal/candida-auris/tracking-c-auris.html). PHD laboratory data also underrepresent rural, smaller hospitals (10), potentially further biasing this convenience sample of C. auris cases. In conclusion, this analysis of hospitalization data supports previous targeted reports and demonstrates a need for strengthened national surveillance and further studies to identify risk factors for C. auris infection and colonization.
Ms. Benedict is an epidemiologist in the Mycotic Diseases Branch, Division of Foodborne, Waterborne, and Environmental Diseases, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA. Her research interests include the epidemiology and prevention of fungal infections.
Nationwide Outbreak of Candida auris Infections Driven by COVID-19 Hospitalizations, Israel, 2021–2022
Roni Biran, Regev Cohen, Talya Finn, Tal Brosh-Nissimov, Galia Rahav, Dafna Yahav, Sharon Amit, Yael Shachor-Meyouhas, Alaa Atamna, Jihad Bishara, Liat Ashkenazi-Hoffnung, Haim Ben Zvi, Mirit Hershman-Sarafov, Shlomo Maayan, Yasmin Maor, Orna Schwartz, Oren Zimhony, Jonathan Lellouche, Meital Elbaz, Ela Burdelova, Naama Mizrahi, Anna Novikov, Oryan Henig, and Ronen Ben-Ami
Abstract
We report an outbreak of Candida auris across multiple healthcare facilities in Israel. For the period of May 2014–May 2022, a total of 209 patients with C. auris infection or colonization were identified. The C. auris incidence rate increased 30-fold in 2021 (p = 0.00015), corresponding in time with surges of COVID-19–related hospitalization. Multilocus sequence typing revealed hospital-level outbreaks with distinct clones.
A clade III clone, imported into Israel in 2016, accounted for 48.8% of typed isolates after January 2021 and was more frequently resistant to fluconazole (100% vs. 63%; p = 0.00017) and voriconazole (74% vs. 5.2%; p<0.0001) than were non–clade III isolates. A total of 23% of patients had COVID-19, and 78% received mechanical ventilation. At the hospital level, outbreaks initially involved mechanically ventilated patients in specialized COVID-19 units and then spread sequentially to ventilated non–COVID-19 patients and nonventilated patients.(SNIP)
Discussion
We report an ongoing nationwide outbreak of C. auris colonization and infection in hospitals in Israel. The introduction of distinct clones of clade I and clade III into 3 hospitals, as well as increased circulation of clade IV, resulted in a 30-fold increase in the annual C. auris incidence rate in 2021. Neither clade I nor clade III had circulated in Israel before 2021, suggesting they arose through importation events into the country. Further, phylogenetic analyses showed that the clade III isolates collected during the current outbreak were related to those imported into Israel from South Africa in 2016 (8). The shift in clade distribution was associated with a change in the azole MIC range; specifically, clade III strains had higher fluconazole and voriconazole MICs compared with those for clade IV strains. Thus, the continuing expansion of clade III in Israel and its spread beyond H1 to other medical facilities might limit the already constrained treatment options for C. auris.
(SNIP)
In summary, C. auris is spreading in multiple hospitals in Israel, and appears set to become endemic in some facilities. The emergence and amplification of new C. auris clones was traced to patients receiving mechanical ventilation in COVID-19 isolation units. C. auris was transmitted from this population to non–COVID-19 patients in shared intermediate care units and from there disseminated to nonventilated patient populations. New guidelines addressing this public health threat were recently published by the Israeli Ministry of Health (27). Continued surveillance and implementation of infection control measures, focusing on debilitated patients and those receiving mechanical respiratory support, are essential to control the spread of C. auris.
Dr. Biran is a resident in internal medicine at the Tel Aviv Sourasky Medical Center, Tel Aviv, Israel. Her primary research interest is the epidemiology of hospital-acquired infections.
Viruses tend to get more attention than bacterial or fungal infections because we've had broad spectrum antibiotics since the 1940s, and early anti-fungal medications we're developed in the 1950s and 1960s. While we have some antiviral medications, they are relatively new and tend to target a narrow range of viral pathogens.
But our armamentarium against bacterial and fungal infections is deteriorating, as increasingly resistant organisms continue to emerge.
Two years ago, in MMWR: Transmission of Pan-Resistant and Echinocandin-Resistant Candida auris in Health Care Facilities, we looked at a report on two un-linked pan-resistant clusters (three cases in Washington, D.C., and two in Texas) among previously untreated patients.
While pan-resistant C. auris has been reported on rare occasions, it has always been associated with patients who had already received, or were receiving, anti-fungal treatments.
As we've discussed often (see CDC: COVID-19 Reverses Progress in Fight Against Antimicrobial Resistance in U.S.), every year we draw a little closer to an oft-predicted `post-antibiotic era', where something as simple as a scraped knee, or elective surgery, could prove deadly.
In the 2019 report, the last year comprehensive healthcare and community data were available to calculate, CDC estimated that more than 2.8 million antimicrobial-resistant infections occur in the U.S. each year, with more than 35,000 people dying as a result.
While I cover AMR topics occasionally in this blog, I can heartily recommend CIDRAP's Antimicrobial Stewardship Project as the best place to learn about the growing global threat of AMR.