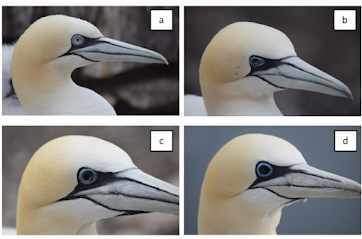
Northern Gannets; a change of eye color from blue to black.
#17,731
While HPAI H5N1's pandemic potential gets most of our attention, its ecological impact - including the deaths of hundreds of millions of captive and wild birds, and the loss of tens (perhaps hundreds) of thousands of mammals - cannot be ignored.
The knock-on effects of these losses may not be fully appreciated for years, but it figures you can't abruptly remove large populations of wildlife from our complex and intertwined eco-system without consequences.
Last summer, in H5N1: The Only Constant is Change, we looked a the continued evolution of the virus, including its diversification into scores of genetically distinct genotypes via reassortment.
The result being some genotypes - or variants within genotypes - may be more transmissible or more pathogenic in some hosts than in others. These changes have enabled the H5N1 virus to spread more efficiently, and over longer distances via migratory birds in recent years, and have greatly expanded the virus's host range (both avian and mammalian).
Sixteen months ago, in DEFRA: The Unprecedented `Order Shift' In Wild Bird H5N1 Positives In Europe & The UK, we looked at the unexpected shift in H5N1 infection from mostly Anseriformes (waterfowl, such as ducks, geese, and swans) to Suliformes and Charadriiformes (shore birds) in 2022.
While some birds (particularly waterfowl) are able to carry the H5N1 virus with few ill effects, this recent shift to sea and shore birds resulted in huge bird die-offs, as these species had little or no preexisting immunity.
Last May, in (Preprint) HPAI (H5N1) in Northern Gannets: Global spread, Clinical signs, and Demographic Consequences, we looked at the horrendous impact H5N1 was having on the Northern Gannet in Europe, and at a curious eye-color change noted among survivors (see photo at top of blog).
But the virus isn't the only thing that changes over time. Survivors of HPAI infection can come away with some degree of immunity. How broadly protective, or long lasting it might be, are open questions.
While obviously a good thing for the individual at-risk host, its impact on the spread and persistence of the virus in the environment is much harder to quantify.
Last last week the UK's APHA and DEFRA published a press release on a new study which finds that some surviving Northern Gannets and Shag are showing signs of developing immunity to H5N1, although they warn:
- With seabirds that have expressed presence of antibodies it remains unclear as to what other long-term effects these birds may experience in terms of reduced life expectancy or lower fertility.
I've posted some excerpts from the press release below, so follow the link to read it in its entirety.
UK study finds some seabirds may develop immunity to bird flu
The eight-strong FluMap consortium, headed by the world-leading research team at the Animal and Plant Health Agency has made a number of bird flu discoveries.
- Scientists have discovered Northern Gannets and Shag are showing signs of developing immunity to avian influenza in research published today.
- The top scientific research consortium has secured £3.3 million additional funding for further research into avian influenza transmission due to continued risk of spread of disease
- New research will explore the evolution of the virus and the ability to predict new strains, protecting both animal and human health.
As part of a major research consortium announced last June, the UK’s top scientists have discovered that some seabirds are demonstrating immunity to avian influenza.
The eight-strong FluMap consortium, headed by the world-leading research team at the Animal Plant Health Agency (APHA), has developed laboratory tools that can dissect the immune response in birds that have been exposed to avian influenza viruses in their lifetime.
Preliminary investigations in a small sample size of some species of seabird, including Northern gannets and Shag, revealed specific immunity to H5N1 showing exposure and recovery in a proportion of birds. However, avian influenza viruses are prone to change and so antibody levels will likely decline over time with next year’s offspring not guaranteed to be immune suggesting there are no great population level benefits yet.
Scientists hope to look at the effect of antibodies on infection, to better predict the emergence of new viruses with different protein combinations in the future, allowing experts to stay one step ahead to safeguard animal and human health, given 60% of new human diseases originate in animals.
High pathogenicity avian influenza (bird flu) is a significant burden on animal health globally and threatens human health, with over 350 infected UK premises detected between 2020 and 2023. Wild bird populations have also suffered significant mortalities across multiple species and wild mammals that have scavenged dead bird carcasses have also been infected.
The consortium has also identified several genetic characteristics that explain the ability of the current H5N1 viruses to spread fast and infect a greater range of species. Research has found that multiple virus genes have switched and evolved to act together to enhance fitness to infect, transmit and persist in birds, but remain un-adapted to humans.
The consortium has mapped the spread of infection over time and made important discoveries regarding airborne transmission of the virus – determining that infectious virus can only travel short distances (less than 10 metres) and is very unlikely between farms through the air.
To further our understanding of this terrible disease, including to study immunity in a range of wild birds, an additional £3.3 million from UK Research and Innovation’s (UKRI) Tackling Infections programme and the Department for Environment, Food and Rural Affairs (Defra) has been granted.
A further £3.2 million has also been allocated for a sister consortium, focussing on the potential for human transmission. Partners from both consortia will work closely together in a One Health approach. A joined-up ‘One Health’ approach aims to sustainably balance and optimise the health of people, animals and the environment, recognising that these things are interdependent. This research will allow for better understanding of the potential impacts of animal-human-environment avian influenza interactions. Cross government community collaboration is key in effectively responding to and preparing for evolving threats like avian influenza.
(SNIP)
The research will be published at 9am tomorrow morning and will be available here:
https://science.vla.gov.uk/fluglobalnet/publications/flumap-update-oct23.html
If it seems as if governments and researchers are suddenly taking a much more serious look at HPAI H5N1, you'd be right. Until 2021, the virus was primarily a threat to poultry, and conventional culling methods, biosecurity measures, and (in some countries) vaccines appeared to be adequate to control it.
But the wheels fell off in 2021, with the emergence of a new and improved H5N1 virus. The virus became endemic in wild and migratory birds, rendering control methods that worked in the past ineffective.
Where things go from here is anyone's guess. We've been on the precipice before, only to see the threat recede. But this time, we appear to have less control over the outcome than we did before.
And that's never a good feeling.