Current Wave of H5N1 reported to FAO
#18,256
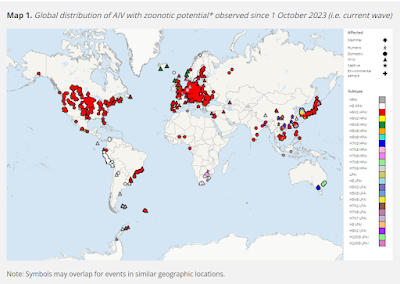
While large swaths of the world are not testing for (or, in some cases, are unwilling to report finding) HPAI H5, those regions actively looking for the virus are finding it in abundance, not only in wild birds, but in many mammalian species.
Only Australia and NZ appear to be currently free of the H5N1 virus (although they have their own homegrown HPAI H7 viruses to contend with).
Importantly for avian flu, very few migratory birds appear to cross the Wallace line (see The Australo-Papuan bird migration system: another consequence of Wallace's Line).
Given movement data demonstrating connectivity between the polar front to both the Antarctic and Subantarctic islands of Oceania, and Oceania itself, it is plausible that if HPAI H5 were present in the Antarctic region directly south of Oceania, it could be introduced to Oceania.
And of course, should H5N1 successfully make the transition to a pandemic virus, those barriers would quickly become irrelevant, as the virus would only be a plane ride away from anywhere in the world.
While the virus still needs to acquire additional mammalian mutations to make that leap, given its unprecedented spread and evolution in recent years, increasingly we are seeing researchers openly discuss the possibility of seeing an H5N1 pandemic.
This variant will continue to evolve, and as it infects more mammalian hosts, it will inevitably continue to acquire mammalian adaptations. This has led to increased concern that HPAIV H5N1 could spill over into humans more efficiently and potentially cause the next human pandemic.
I've only posted the link and some excerpts, so follow the link to read it in its entirety. I'll have a brief postscript after the break.
RESEARCH ARTICLE (Open Access)
High pathogenicity avian influenza in Australia and beyond: could avian influenza cause the next human pandemic?
Megan Airey A and Kirsty R. Short A * * Correspondence to: k.short@uq.edu.au
Microbiology Australia https://doi.org/10.1071/MA24040
Submitted: 18 June 2024 Accepted: 4 August 2024 Published: 19 August 2024
© 2024 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the ASM. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
The primary natural reservoir for avian influenza viruses is wild waterfowl. In poultry, some of these viruses can evolve into high pathogenicity avian influenza viruses (HPAIVs) that cause significant disease. HPAIV H5N1 clade 2.3.4.4b is a current variant of concern that has caused mass die-offs of wild birds, land and marine mammals all across the world since its emergence in 2020.
This article explores the history associated with HPAIVs, as well as the current global situation pertaining to HPAIV H5N1 clade 2.3.3.4b and the HPAIV situation in Australia. This variant will continue to evolve, and as it infects more mammalian hosts, it will inevitably continue to acquire mammalian adaptations. This has led to increased concern that HPAIV H5N1 could spill over into humans more efficiently and potentially cause the next human pandemic
(SNIP)
The global situation
The newest variant of HPAIV H5N1 clade 2.3.4.4b was first detected in the Netherlands in late 2020.9 This variant quickly circulated throughout Europe and was detected within poultry in Newfoundland and Labrador, Canada, and shortly thereafter, within the United States. The next year, it was introduced to South America.10 Unfortunately, by late 2022, clade 2.3.4.4b had reached the bottommost tip of South America, posing a serious risk of transmission to Antarctica.10
HPAIV H5N1 clade 2.3.4.4b, like all HPAIVs, is associated with high morbidity and mortality within poultry,9,10 and has caused mass poultry die offs across Europe, Africa, Asia and the Americas over the past few years.11 By 2023, it was estimated that this variant had caused the death of 58 million birds in the US alone.12 Unusually, HPAIV H5N1 2.3.4.4b has also caused die offs of a vast range of wild birds,8 even solitary birds such as bald eagles have presented with severe disease.13
Several mammalian species including racoons, foxes, dogs and cats, have also been infected, likely by predation or scavenging of infected birds.14 Within South America, ~50,000 marine mammals, including seals, sea lions and dolphins have been killed by the virus off the coast of Peru, Argentina, Chile and Uruguay.15 Symptoms were mostly neurological, i.e. seizures, paralysis and stupor; however, respiratory symptoms such as nasal or buccal secretions and dyspnoea were also documented.15 The infection of marine mammals and birds in South America represents a threat to the unique ecological niche of Antarctica. There have been many outbreaks within elephant and fur seals, and birds including Gentoo penguins, kelp gulls, albatross and skuas within the continent and its islands.16
In April 2024, the US Department of Agriculture (USDA) confirmed the detection of HPAIV H5N1 clade 2.3.4.4b within a series of dairy herds in several states including Texas, Michigan and Kansas.1,17 As of July 2024, 171 herds within 13 US states have detected the variant,1 with reported viral titres within unpasteurised milk between 2 × 105 to 6 × 107 plaque-forming units (PFUs) mL–1.18 Notably, pasteurisation inactivates the virus, preventing transmission by milk consumption.18 Mice and barn cats on these farms have also been infected with the variant likely from consuming unpasteurised milk, with many of these cases in cats proving lethal.2 This outbreak represents a new facet of HPAIV H5N1 transmission and pathogenesis, with ongoing implications for the dairy industry, human and animal health.
Human spillover of HPAIV H5N1 clade 2.3.4.4b is very rare – as of June 2024, there have been 15 human cases reported within seven countries (Cambodia, Chile, China, Ecuador, Spain, the US and the UK).19 However, other HPAIV H5N1 variants have exhibited a high mortality rate – HPAIV H5N1 clade 2.3.4.4b has caused the death of one person in China in 2022.19 The majority of these human cases were a result of direct exposure to infected poultry, and importantly, no evidence of human-to-human transmission has ever been identified.19
However, four dairy workers have tested positive for HPAIV H5N1 clade 2.3.4.4b by exposure to infected dairy cows,1,20 suggesting this variant may be transmitted from mammal-to-mammal without the involvement of an infected wild bird. Most of these cases among dairy workers were mild20; however, it is noted that this number could be an understatement due to the high likelihood of underreporting on dairy farms across America. Symptoms in humans include but are not limited to conjunctivitis, fever, body aches, cough, sore throat and, in severe cases, pneumonia that requires hospitalisation.19 However, 7 of 15 of these HPAIV H5N1 clade 2.3.4.4b human cases have also been reported as asymptomatic.19
(SNIP)
HPAIV H5N1: the next human viral pandemic?
Influenza A viruses are considered a significant threat to public health due to their high mutation rate and pandemic potential.30 There have been several past influenza A pandemics in humans, including the 1918 H1N1 ‘Spanish flu,’ the 1957 H2N2 ‘Asian flu,’ the 1968 H3N2 ‘Hong Kong flu’ and the 2009 H1N1 ‘Swine flu’.31 Currently, HPAIV H5N1 transmission to humans requires direct, close contact with an infected host, such as wild birds or poultry.32 HPAIV H5N1 can spread by aerosolised droplets and preferentially binds to α-2,3-sialic acids found within the lower respiratory tract of humans.32
As avian influenza viruses begin to become more adapted to the mammalian system, the acquisition of mammalian adaptations by reassortment or antigenic drift may increase the risk of transmission to humans.30
Human-to-human transmission of HPAIV H5N1 has not been confirmed; however, there is some evidence to suggest that sustained mammal-to-mammal transmission has occurred within marine mammals in South America.33 Sequencing of virus samples obtained from sick and deceased sea lions within these areas demonstrates several key mutations important for mammalian transmission.33 Some of these include mutations PB20-Q591K and PB2-D701N, both known to increase pathogenicity by enhancing the activity of the polymerase basic 2 (PB2) protein within mammals.34 Bioinformatic analysis and machine learning algorithms must be trained and implemented to evaluate the pandemic potential of HPAIV H5N1 clade 2.3.4.4b as it evolves.
Conclusions
Globally, the HPAIV H5N1 situation will continue to change. The lack of human-to-human transmission of the virus is encouraging. However, we are also witnessing the spread of this virus into previously uninfected continents and species. To better stand prepared against HPAIV H5N1 clade 2.3.4.4b, we must continue to uphold a series of strict biosecurity measures on poultry farms with confirmed cases of HPAIV,35 especially as wild birds migrate back to Australia between August and November 2024.35
If this variant were to be introduced into Australia, it would cause devastation to our unique ecosystem and species diversity.36 Many Australian native birds have never been exposed to this virus before,36 and some birds, including black swans, are known to be highly susceptible to severe disease from avian influenza virus.37
Furthermore, the potential for sustained human-to-human transmission of HPAIV H5N1 clade 2.3.4.4b is still of great concern, especially as mammalian transmission of the virus continues to occur.
However, several strategies including enhanced surveillance, stockpiling of antivirals and vaccines and the production of a novel vaccine must be prioritised by governments now to help mitigate the severity of any future pandemic.
Whether or not H5N1 has what it takes to spark a human pandemic remains unknowable, but it is far from the only zoonotic threat in the queue.
We now live in an age (see The Third Epidemiological Transition) where the the number, frequency, and intensity of pandemics are only expected to increase over the next few decades.The CDC currently has 25 influenza A viruses on their IRAT list, and the WHO recently released a 38-page Pathogens Prioritization report that adds dozens of non-influenza zoonotic threats to their watch list.
While we can debate when - or from what - another pandemic is inevitable. All we can really control is how well prepared we are, when that happens.