#18,793
History has shown that influenza pandemics can vary widely, both in severity and in demographic impact. The 1918 H1N1 pandemic was not only high-severity, it was reportedly far deadlier to healthy young adults than to the elderly (see epi curve above).
In 2010, (see Study: Years Of Life Lost Due To 2009 Pandemic), researchers estimated the median age of death due to seasonal influenza-related illness in the United States to be 76.
`Half of influenza-related deaths during the 1968-1969 influenza A (H3N2) pandemic and large proportions of influenza-related deaths during the 1957-1958 influenza A (H2N2) and the 1918-1919 influenza A (H1N1) pandemics occurred among persons <65 years old.'
While the 2009 H1N1 pandemic was milder, we saw an even greater age shift. The CDC’s estimate of average and median age of death due to the 2009 Pandemic virus reads:
Based on two CDC investigations of confirmed 2009 H1N1-related deaths that occurred during the spring and fall of 2009, the average age of people in the U.S. who died from 2009 H1N1 from April to July of 2009 was 40. The median age of death for this time period was 43. From September to October of 2009, the average age of people in the U.S. who died from 2009 H1N1 was 41, and the median age was 45.
Bucking these trends, the 5-year epidemic of H7N9 in China had its greatest impact on older adults and the elderly (see "Avian influenza A (H7N9) virus infections in humans across five epidemics in mainland China, 2013–2017). The authors wrote:
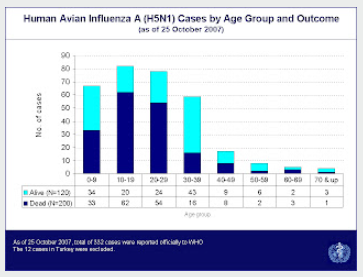
Yet H5N1, which is currently high on our watch list, has consistently skewed towards a younger demographic. Eighteen years ago, in A Predilection For The Young, I wrote about the disturbing skewing of H5N1 cases (and deaths) among younger individuals (see WHO Chart below).A linear increase in fatality risk was observed from the younger age groups to older age groups (Figure 4B). Case-patients aged over 60 years consistently had higher risks for death, death/mechanical ventilation, death/mechanical ventilation/ICU admission than case-patients aged below 60 years.
Over the past decade it as become increasingly apparent that the first influenza virus you are exposed to shapes your immune response to other subtypes later in life (see Nature: Declan Butler On How Your First Bout Of Flu Leaves A Lasting Impression).
While the subtype (H1, H2, H3, etc.) is often assumed to have the biggest impact, the HA Group type (see chart below) can also drive future immune responses (see Science: Protection Against Novel Flu Subtypes Via Childhood HA Imprinting).
Obviously, knowing what cohorts or risk groups would be most vulnerable in a future influenza pandemic would greatly aid in preparedness, and in the allocation of vaccines and antivirals.
But, as today's preprint by Danuta M. Skowronski et al. reports that immune imprinting may depend on more than just the first HA exposure, as the first NA (neuraminidase) may play a significant role as well.This is a fascinating report, which adds both H5N6 and H9N2 to the equation. Despite sharing a near-identical HA with H5N1, H5N6 consistently skews towards an older demographic.
H9N2, on the other hand, skews to an even younger cohort than H5N1.
This paper posits that the lower attack rate and CFR from H5N1 over the past 15 years may be due to the `original pediatric priming' of children from the NA of the 2009 H1N1 pandemic, and a `massive boost opportunity' for older cohorts who were first exposed to H1N1 (born prior to 1957).
The authors also suggest that while this anti-NA immunity may help moderate the severity of both H5N1 and H9N2, it may also represent a double-edge sword. They write:
Homosubtypic anti-NA in particular may have attenuating effects on H5N1 and H9N2 zoonotic risk, notably severity, with implications for targeted messaging and mitigation measures.
Alternatively, while beneficial to those directly exposed, anti-NA may also paradoxically contribute to unrecognized infections, potentially facilitating surreptitious shedding, spreading and viral adaptation, with implications for case detection, containment and enhanced surveillance.There is a lot to unpack here, and you'll want to download and read the full report. I'll have a bit more after the break.
Neuraminidase imprinting and the age-related risk of zoonotic influenza
While it is conceivable that some degree of community immunity to the NA of H5N1 might help blunt any future impact, it is far from guaranteed. Influenza A is a notoriously mutable virus, and H5 has swapped out its NA gene repeatedly over the years.
H5N8 dominated between 2014-2020, we continue to see spillovers of H5N6 viruses to humans in China, and over the past two years we've seen an increasing number of detections of H5N5 in mammalian wildlife in Canada and Northern Europe.Although it also carries an N1 neuraminidase gene, the older H5N1 clade circulating in Cambodia continues to produce an impressive 30%-40% case fatality rate. Granted, its N1 is structurally different from clade 2.3.4.4b, but we've already seen them reassort.
An H5N1 pandemic is far from assured, and we could easily be blindsided by something else. Predicting the next pandemic is a mug's game.