# 3401
In my post-paramedic career I was an estimator and project manager on a number of large commercial construction projects.
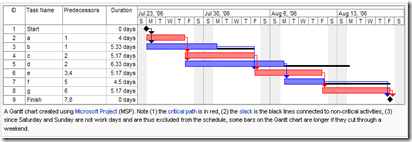
One of the skills you learn is Critical Path Analysis and the use of PERT (Project Evaluation and Review Technique) and Gantt Charts (a bar chart that illustrates a project schedule)
A sample Gantt chart is depicted below, followed by a PERT chart.
These complex appearing charts can often mean the difference between success and failure of an entire project.
A few years later, when I became a computer consultant and custom software programmer, I found myself using the same techniques when I was designing large software projects.
In any complex project there are steps, or actions, that can only be taken once other steps have been completed, while others can run in parallel.
When building a house, you can’t pour the slab until the plumber has roughed in the plumbing and the building inspector has signed off on it.
But you can schedule the drywall and the landscaper at the same time. And you can have the roofer putting on shingles while the electrician is roughing in the wiring.
Of course, you could just wait and do each item in sequence. But you lose precious days, weeks, or even months that way, and for most projects, that would mean prohibitive cost overruns.
Which is why the CPM (Critical Path Method) is so important.
And when you are talking about rolling out a massive vaccination program against a novel influenza, wasted time could cost many lives.
First, a news article about the proposed vaccination program for the United States, and then some thoughts about the critical path to getting it done.
Massive Swine Flu Vaccination Campaign Being Discussed
Posted on: Saturday, 27 June 2009, 06:35 CDT
According to officials, an unprecedented 600 million doses of swine flu vaccine may be given this fall even though officials have not yet developed a way to administer such a large number of shots.
The campaign would be much larger than the normal 115 million doses of seasonal flu vaccine administered each year, said officials at a national vaccine advisory committee meeting.
Currently, no decision has been made as to whether the swine flu vaccination will take place.
According to health officials, the campaign could be here soon.
As many as 60 million doses of the vaccine could be ready for distribution by September, if the vaccine can be tested and produced.
Despite the unknowns, officials are preparing for a massive vaccination campaign, and are fearful that the illness could accelerate by winter. Discussions on the massive campaign dominated a three-day meeting of the Advisory Committee on Immunization Practices, which guides the U.S. on vaccination policies.
(Continue . . .)
Despite the `forward looking’ statements about delivering 600 million doses of vaccine to Americans this fall, an awful lot must happen before that can become even a remote possibility.
The projection of 60 million doses of vaccine ready for distribution in September is also highly speculative, given that we don’t have any animal or human studies yet, and we don’t know how much antigen will be required to invoke an immune reaction.
Until we know that, we can’t really know how many doses will be available, or when.
The CDC, to their credit, has continually resisted giving any timetable for the American public seeing mass vaccinations against the pandemic flu.
Although the first batches of vaccine have been ordered for about 20 million critical infrastructure workers, the decision to manufacture enough vaccine for the entire nation has not been made.
Why not?
First, until we have animal and human studies showing that this pandemic vaccine is safe and effective, you really don’t want to be ordering up 600 million doses of an unproven vaccine.
Second, until we know how much antigen is required (and whether it will take 1 or 2 shots per person), we don’t even know how much vaccine to order.
There’s a big difference between needing 300 million doses and 600 million.
Third, scientists are waiting as long as possible before ordering the bulk of the vaccine because they fear the virus could mutate over the summer, which might make any vaccine produced now less effective in the fall.
Fourth, we honestly don’t know how long it will take to produce enough vaccine for 600 (or even 300) million doses.
Suggesting that it could all be done `by fall’, as the above article does - given the global competition for available vaccine production runs - would seem to be more than a little optimistic.
Fifth, authorities aren’t quite sure how they can deliver up to 600 million doses of vaccine (assumes 2 shots, 3 weeks apart) to 300 million people in a short period of time.
And that’s on top of administering roughly 120 million seasonal flu shots.
It’s never been done before.
The last time it was tried, in 1976, it ended up being a public health debacle (see Deja Flu, All Over Again). No one is anxious to see a repeat of that fiasco.
Health Departments around the country have been hit hard by budget cuts over the past few years, and no one knows where the money and personnel will come from to accomplish this monumental task.
And assuming that high risk groups will be prioritized to receive the vaccine, that will add another layer of complexity to the program.
Tracking recipients, and possible adverse effects, adds another wrinkle.
With 50 states, and thousands of county health departments involved, there are an incredible number of logistical problems involved in any national vaccination program.
And Sixth, it isn’t clear how many Americans would be willing to take a swine flu vaccine.
The constant reminders from many officials that this virus is `relatively mild’ and `no worse than seasonal flu’ may come back to haunt officials in the fall, should they decide to push the vaccine. Especially if the virus picks up virulence.
How much sway the anti-vaccine crowd and the conspiratorial websites will have, is also unknown. But you can bet that they, and the media, will play up any reports of side effects from the vaccine.
Selling the idea that – in addition to a seasonal vaccine, people need to get two pandemic jabs – isn’t going to be easy.
The serpentine route to developing and deploying a vaccine to an entire nation is fraught with numerous twists and turns, and no one can foresee them all in advance.
I certainly can’t.
And even armed with the best PERT and Gantt charts they can devise, the planners in our public health departments can’t either.
In an extraordinarily complex operation such as this, there are simply too many opportunities for problems to arise, and delays to occur. Which is why the CDC is wisely refraining from making promises regarding delivery of a vaccine.
Despite these (and many other) obstacles, I remain in favor of developing and deploying a swine flu vaccine this fall.
I bring up these issues, not to discourage a vaccine, but to make sure people have a realistic idea of what they can . . . and can’t . . . expect this fall.
I fully expect that, barring some major problem in the clinical trials, some quantity of pandemic vaccine will be available this fall. I further expect that over the winter, more vaccine will become available.
How much?
I don’t think anyone knows right now. Hopefully enough to protect those at greatest risk from this virus.
But I wouldn’t put much money on the idea that – as the above article suggests – 600 million doses of vaccine could be given to all 300 million Americans this fall.
Call me a cynic, but I just don’t see a path that gets that done by the end of this year.