#16,932
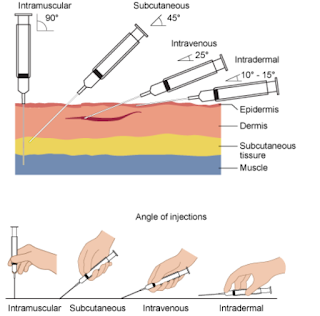
The JYNNEOS Monkeypox vaccine - which was approved in 2019 - remains in short supply while demand continues to soar. While originally designed to be administered as a subcutaneous injection (see package insert below), the FDA has approved a new, dose-sparing intradermal injection which could increase the number of people who can be vaccinated 5-fold.
The rationale for this change is based on the results of a single 2015 study which showed that 2 fractional doses (1/5th) of JYNNEOS given intradermally, 28 days apart, provided similar protection as that seen from the original route.
First the announcement from the FDA, after which I'll have a brief postscript.Monkeypox Update: FDA Authorizes Emergency Use of JYNNEOS Vaccine to Increase Vaccine Supply
For Immediate Release:August 09, 2022
Today, the U.S. Food and Drug Administration issued an emergency use authorization (EUA) for the JYNNEOS vaccine to allow healthcare providers to use the vaccine by intradermal injection for individuals 18 years of age and older who are determined to be at high risk for monkeypox infection. This will increase the total number of doses available for use by up to five-fold. The EUA also allows for use of the vaccine in individuals younger than 18 years of age determined to be at high risk of monkeypox infection; in these individuals JYNNEOS is administered by subcutaneous injection.
“In recent weeks the monkeypox virus has continued to spread at a rate that has made it clear our current vaccine supply will not meet the current demand,” said FDA Commissioner Robert M. Califf, M.D. “The FDA quickly explored other scientifically appropriate options to facilitate access to the vaccine for all impacted individuals. By increasing the number of available doses, more individuals who want to be vaccinated against monkeypox will now have the opportunity to do so.”
JYNNEOS, the Modified Vaccinia Ankara (MVA) vaccine, was approved in 2019 for prevention of smallpox and monkeypox disease in adults 18 years of age and older determined to be at high risk for smallpox or monkeypox infection. JYNNEOS is administered beneath the skin (subcutaneously) as two doses, four weeks (28 days) apart. For individuals 18 years of age and older determined to be at high risk of monkeypox infection, the EUA now allows for a fraction of the JYNNEOS dose to be administered between the layers of the skin (intradermally). Two doses of the vaccine given four weeks (28 days) apart will still be needed. There are no data available to indicate that one dose of JYNNEOS will provide long-lasting protection, which will be needed to control the current monkeypox outbreak.
Data from a 2015 clinical study of the MVA vaccine evaluated a two-dose series given intradermally compared to subcutaneously. Individuals who received the vaccine intradermally received a lower volume (one fifth) than individuals who received the vaccine subcutaneously. The results of this study demonstrated that intradermal administration produced a similar immune response to subcutaneous administration, meaning individuals in both groups responded to vaccination in a similar way. Administration by the intradermal route resulted in more redness, firmness, itchiness and swelling at the injection site, but less pain, and these side effects were manageable. The FDA has determined that the known and potential benefits of JYNNEOS outweigh the known and potential risks for the authorized uses.
To support the FDA’s authorization of two doses of JYNNEOS administered by the subcutaneous route of administration in individuals younger than 18 years of age, the FDA considered the available JYNNEOS safety and immune response data in adults as well as the historical data with use of live vaccinia virus smallpox vaccine in pediatric populations.
JYNNEOS has been tested in individuals with immunocompromising conditions and has been found to be safe and effective in the trials that were performed to support approval. It was initially developed specifically as an alternative for use in immunocompromised individuals in the event of a smallpox outbreak.
On the basis of the determination by the Secretary of the Department of Health and Human Services on Aug. 9, 2022, that there is a public health emergency, or the significant potential for a public health emergency, that has a significant potential to affect national security or the health and security of United States citizens living abroad, and the declaration on Aug. 9, 2022, that circumstances exist justifying the emergency use of vaccines, the FDA may issue an EUA to allow emergency use of unapproved vaccines or unapproved uses of approved vaccines.
The FDA will provide updates as developments occur and will continue to work with federal public health partners and industry to ensure timely access to all available medical countermeasures. More information can be found on the agency’s monkeypox webpage.
For more than a decade researchers have been warning that the risk from Monkeypox was rising, primarily due to our declining immunity to smallpox. Routine vaccination for smallpox - which is believed to have provided about 85% protection against Monkeypox - was discontinued in the 1970s due to the eradication of the disease.
Today, with few exceptions, only those over the age of 50 are vaccinated. And the durability of protection afforded by that vaccine after 50+ years is unknown.
Vaccine Effectiveness
Because Monkeypox virus is closely related to the virus that causes smallpox, the smallpox vaccine can protect people from getting monkeypox. Past data from Africa suggests that the smallpox vaccine is at least 85% effective in preventing monkeypox. The effectiveness of JYNNEOS(TM) against monkeypox was concluded from a clinical study on the immunogenicity of JYNNEOS and efficacy data from animal studies.
Smallpox and monkeypox vaccines are effective at protecting people against monkeypox when given before exposure to monkeypox. Experts also believe that vaccination after a monkeypox exposure may help prevent the disease or make it less severe.
With this FDA announcement, the current supply of JYNNEOS vaccine (est. 400,000 remaining doses) can be stretched to roughly 2 million. And given the speed at which Monkeypox is spreading, those additional doses will almost certainly be needed.