Download this book for free at http://open.bccampus.ca,
CC BY 4.0, via Wikimedia Commons
#16,957
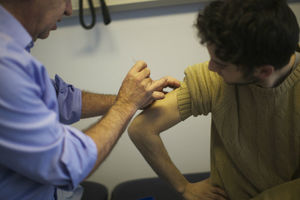
Faced with a limited supply of the JYNNEOS (TM) Smallpox and Monkeypox Vaccine, two weeks ago the FDA Issued an EUA Allowing `Dose-sparing' Intradermal Injections that would stretch existing vaccine supplies as much as 5-fold.
Called `Fractional Dosing', this could allow far more people to be protected in the short term, while more vaccine is produced.
The rationale for this change is based on the results of a single 2015 study which showed that 2 fractional doses (1/5th) of JYNNEOS given intradermally, 28 days apart, provided similar protection as that seen from the original route.
Last week the European Medicines Agency (EMA) followed suit, issuing
EMA’s Emergency Task Force advises on intradermal use of Imvanex / Jynneos against monkeypox(Continue . . . )
News 19/08/2022
EMA’s Emergency Task Force (ETF) has reviewed data on the monkeypox vaccine Imvanex 1 used as an intradermal injection (given just below the top layer of the skin).
The vaccine is only authorised for subcutaneous injection (injection under the skin). However, when given intradermally, a smaller dose of the vaccine can be used. Given the currently limited supply of the vaccine, this means that more people can be vaccinated.
The ETF reviewed data from a clinical trial 2 involving around 500 adults, which compared the vaccine given either intradermally or subcutaneously, as 2 doses with a 4-week interval between each dose. People receiving the vaccine intradermally received one fifth (0.1 ml) of the subcutaneous dose (0.5 ml) but produced similar levels of antibodies to those who received the higher subcutaneous dose.
The ETF cautioned that there was a higher risk of local reactions (e.g. longer-lasting redness, and thickening or discoloration of the skin) after intradermal injections.
The ETF noted that there is no information available on the maximum number of 0.1 ml doses that can be withdrawn from the authorised presentation (0.5 ml suspension) and recommended using low-dead volume syringes to optimise the number of doses that can be extracted. The ETF also emphasised the importance of giving intradermal injections correctly, recommending that only healthcare professionals with experience giving injections intradermally should administer the vaccine this way. Further information for healthcare professionals is included in the ETF’s statement.
Taking into account all these considerations, national authorities may decide as a temporary measure to use Imvanex as an intradermal injection at a lower dose to protect at-risk individuals during the current monkeypox outbreak while supply of the vaccine remains limited.
Yesterday the UK's Health Security Agency (UKHSA) announced that fractional dosing would be trialed at 3 clinics in the UK, in order to evaluate its performance.
Press release
Monkeypox vaccines to be piloted in smaller but equally effective doses
Eligible patients offered smaller but equally effective doses of the vaccine, stretching existing supplies to protect more people.
From:UK Health Security Agency Published 22 August 2022
Three NHS sites are set to begin a pilot offering eligible patients smaller but equally effective doses of the vaccine used for the UK’s monkeypox outbreak, stretching existing supplies to protect more people.
The safe and clinically-approved approach, known as ‘fractional dosing’, has been commonly used in other worldwide outbreaks when vaccine supplies are constrained. It will be introduced in one sexual health clinic in Manchester from today (Monday), and a further 2 in London shortly.
Fractional dosing could maximise the number of doses that can be administered without compromising protection, with clinical study results showing it provides a near-identical immune response in patients.
Under the approach, eligible people aged 18 and over will be offered a 0.1ml dose of the smallpox Jynneos vaccine, instead of the 0.5ml dose that is typically administered. This will potentially enable up to a 5-fold increase in the number of people that can be offered vaccination.
Fractional dosing has recently been authorised in the US by the Federal Drug Administration for its own monkeypox response. The European Medicines Agency Emergency Task Force has also approved the approach.
The UK Health Security Agency (UKHSA) has reviewed the evidence in detail alongside the Joint Committee on Vaccination and Immunisation (JCVI) and is now working with NHS England to test the feasibility of the approach at pilot clinics in Chelsea and Westminster NHS Foundation Trust, Central and North West London NHS Foundation Trust, and Locala Health and Wellbeing in Greater Manchester.
In a letter to Directors of Public Health, UKHSA chief executive Professor Dame Jenny Harries has confirmed the details of the pilot, with data gathered by the clinics used to inform planning for possible wider use when more doses of the vaccine arrive in the UK.
Vaccine Effectiveness
Because Monkeypox virus is closely related to the virus that causes smallpox, the smallpox vaccine can protect people from getting monkeypox. Past data from Africa suggests that the smallpox vaccine is at least 85% effective in preventing monkeypox. The effectiveness of JYNNEOS(TM) against monkeypox was concluded from a clinical study on the immunogenicity of JYNNEOS and efficacy data from animal studies.
Smallpox and monkeypox vaccines are effective at protecting people against monkeypox when given before exposure to monkeypox. Experts also believe that vaccination after a monkeypox exposure may help prevent the disease or make it less severe.
For more than a decade researchers have been warning that the risk from Monkeypox was rising, primarily due to our declining immunity to smallpox. Routine vaccination for smallpox - which is believed to have been substantially protective against Monkeypox - was discontinued in the 1970s due to the eradication of the disease.
Today, with very few exceptions, only those over the age of 50 are vaccinated against smallpox. And the durability of protection afforded by that vaccine after 50+ years is unknown.
With limited JYNNEOS vaccine availability - and an uncertain trajectory for the global Monkeypox epidemic - data gleaned from the UK's pilot program could help determine how to best use the limited supply expected to be produced over the next year.