Credit CDC
#17,432
In June of 2016 the CDC issued a Clinical Alert to U.S. Healthcare facilities about the Global Emergence of Invasive Infections Caused by the Multidrug-Resistant Yeast Candida auris. A week later we saw a report from the UK's PHE on an outbreak that had been ongoing their since 2015 (see PHE On The Emergence Of Candida auris In The UK).
Unlike most systemic Candida infections, which usually arise when a previously colonized person is weakened from illness or infirmity, this strain has a propensity for nosocomial transmission.C. auris is an emerging fungal pathogen that was first isolated in Japan in 2009. It was initially found in the discharge from a patient's external ear (hence the name `auris'). Retrospective analysis has traced this fungal infection back over 20 years.
When you add in that:
- C. auris infections have a high fatality rate
- The strain has developed resistance to multiple classes of anti-fungals
- This strain is unusually persistent on fomites in healthcare environments.
- And it can be difficult for labs to differentiate between Candida strains
MMWR: Transmission of Pan-Resistant and Echinocandin-Resistant Candida auris in Health Care Facilities
mBio: On the Emergence of Candida auris: Climate Change, Azoles, Swamps, and Birds
mSphere: Comparative Pathogenicity of UK Isolates of the Emerging Candida auris
While the COVID pandemic has driven C. auris from the front pages and disrupted surveillance, this MDRO (Multidrug-Resistant Organism) continues its spread (see CDC: COVID-19 Reverses Progress in Fight Against Antimicrobial Resistance in U.S.).
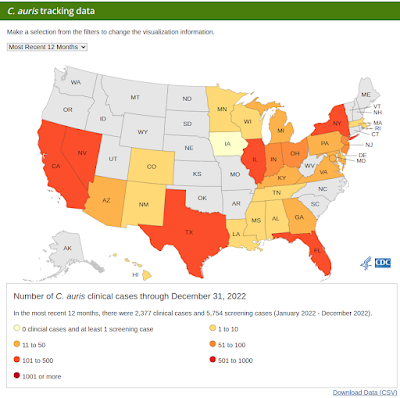
Surveillance remains spotty, and the most recent numbers on the CDC's website (below) only go through Dec 31st, 2022. C. auris infection (and asymptomatic colonization) are significantly under-reported, both here in the United States and around the globe.
Now that the COVID emergency has declined, more attention is being focused again on C. auris, and other MDROs, such as C. difficle, CRE, HvKp and MRSA. This past week, The Lancet published the following cautionary commentary on C. auris.
Candida auris: an emerging antimicrobial-resistant organism with the highest level of concern
Shyam Kumar Mishra Muhammad Yasir Mark Willcox
Open Access Published:April 26, 2023
DOI:https://doi.org/10.1016/S2666-5247(23)00114-3
Candida auris is an emerging multidrug-resistant oval-shaped fungus causing life-threatening health-care-associated outbreaks. This fungal pathogen has been rated as an urgent threat by the US Centres for Disease Control and Prevention and classified in the Critical Priority group by WHO. Since the fungus' discovery in 2009 from the external ear discharge of a female patient aged 70 years in Japan, it has been reported in over 47 countries worldwide.1 A report2 from a multicentre retrospective case-control study from three hospitals in Brooklyn, New York (NY, USA), on the outcomes of echinocandin-susceptible C auris bloodstream infection reported that the 30-day and 90-day mortality rates were 30·1% and 44·6%, respectively.
C auris are potent nosocomial pathogens that can survive in hospitals and on the surface of medical devices. Multidrug-resistant C auris can survive for at least 2 weeks on plastic surfaces and in the viable but non-culturable state for at least 4 weeks.3 The most common risk factors for the acquisition of C auris infection are the use of invasive devices and being immunocompromised. C auris can colonise patients showing predilection for the skin. The fungus can contaminate surfaces and hospital equipment, which makes it unique among Candida spp. C auris' armamentaria include the production of biofilm, phospholipase, proteinase, and secreted proteases.4
The rise of C auris infections could be partly attributed to the worldwide increase in the use of antifungals; climate change selecting for thermotolerant yeasts; the fungus' ability to spread in different species; probable gaps in infection control; the acute global shortage of health-care workers and equipment; and an increase in the number of people with debilitating COVID-19, treated with antimicrobials. Circulating strains of C auris have been categorised into different genomic clades: I (in southern Asia), II (in eastern Asia), III (in Africa), IV (in South America), and V (in Iran), each with independent emergence.5
In the past, C auris might have been misidentified as Candida haemulonii. Now, C auris can be identified by PCR amplification of genetic loci such as the D1/D2 region of 28S rDNA and internal transcribed spacer rDNA, or by matrix-assisted laser desorption ionization time of flight mass spectrometry using databases containing all clades of C auris.5
Multidrug-resistant and pandrug-resistant C auris isolates are being increasingly detected worldwide. In the USA, the resistance rate of these fungal pathogens has been found to be 90% against fluconazole, and 30% against amphotericin B, and is increasing over time.6 Echinocandins are preferred for the treatment of C auris infections but increasing resistance to this class of antifungal has been reported.4, 6
Several new strategies, including the development of novel antifungals and combinations of different drugs, need to be developed to treat multidrug-resistant C auris. Potential new antifungals are in various stages of clinical testing, including fosmanogepix, with its active moiety, manogepix, which inhibits glycosylphosphatidylinositol biosynthesis;1 rezafungin, a long lasting echinocandin; ibrexafungerp, a glucan synthase inhibitor; MYC-053, which inhibits intracellular nucleic acid synthesis and synthesis of cell wall chitin; and VT-1598, which inhibits fungal lanosterol demethylase.7 Combinations of micafungin and amphotericin B;8 the serine palmitoyltransferase inhibitor myriocin with flucytosine;9 or isavuconazole plus anidulafungin10 can be effective. Antimicrobial stewardship to reduce unnecessary use of antifungals should also be implemented. People at high risk of infection, such as health-care workers in close contact with C auris infections, should be screened for C auris regularly. Transmission-based precautions should always be practiced: alcohol-based hand sanitisers, hydrogen peroxide, chlorine-based disinfectants, and iodine-based skin antiseptics are effective against C auris.4
In conclusion, to control the rapidly spreading infections due to C auris, laboratories must implement identification methods for their diagnosis. Breakpoints of commonly used antifungals should be defined. New antifungals or combination treatments need to be rapidly identified. C auris infections must be studied from the One Health perspective to understand their epidemiology and to control their spread.
While antibiotics and antifungals still work today for most infections, for tens of thousands of people every year, that `post-antibiotic era' is already here.
While I cover AMR topics from time to time in this blog, I can heartily recommend CIDRAP's Antimicrobial Stewardship Project as the best place to learn about the growing global threat of AMR.