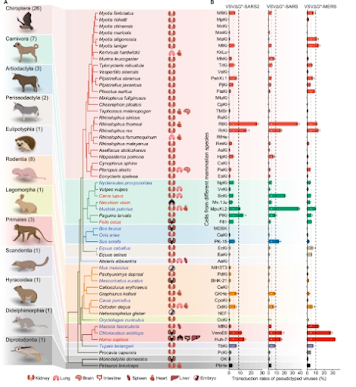
While the SARS-CoV-2 virus originated in an (as yet, unknown) animal host, and has shown a limited ability to infect a wide range of mammals (see USDA Chart below) - it is exquisitely adapted to humans - having infected billions of people (killing millions) over the past 3+ years.
Early in the pandemic we saw reassuring reports that COVID wasn't particularly well suited to infect non-human species (see Susceptibility of Ferrets, Cats, Dogs & Other Domestic Animals to SARS-CoV-2), and mice were considered unsuitable for laboratory studies because they were largely immune.
In late 2020 we saw SARS-CoV-2 jump from humans to farmed mink in Denmark, spread like wildfire, mutating into new mink-variants (see Denmark Orders Culling Of All Mink Following Discovery Of Mutated Coronavirus), several of which spilled back into the community.But over time, just as the SARS-CoV-2 virus has evolved to become more transmissible in humans, it has expanded its host range (see 2021's PrePrint: The B1.351 and P.1 Variants Extend SARS-CoV-2 Host Range to Mice).
This emergency was short-lived as the Alpha variant emerged in Europe in late 2020 and quickly supplanted these mink variants. But other spillovers continued to occur, including to North American White-tailed deer (see 2021's USDA/APHIS: White-Tailed Deer Exposed To SARS-CoV-2 Detected In 4 States).
Deer appear to be infected asymptomatically, and seem capable of transmitting the virus to other deer, but their overall threat remained unquantified.
Subsequent research (see Preprint: Evolutionary Trajectories of SARS-CoV-2 Alpha and Delta Variants in White-Tailed Deer in Pennsylvania) has shown that deer are not only a reservoir for older variants of COVID, they are providing the virus new evolutionary pathways to follow.
Today, variants of SARS-CoV-2 circulate - alongside MERS-CoV, and other coronaviruses - in dozens of species (see Nature: Comparative Susceptibility of SARS-CoV-2, SARS-CoV, and MERS-CoV Across Mammals).
Last week a preprint (see First Eurasian cases of SARS-CoV-2 seropositivity in a free-ranging urban population of wild fallow deer) confirmed the detection of COVID antibodies in deer outside of North America (Ireland), while last May we saw a study finding evidence of SARS-CoV-2 in farmed poultry, cattle, goats in Southern Nigeria.
The concern is that new, and potentially more dangerous variants could arise in a non-human host, and then spill back over into humans. Depending on its virulence and transmissibility, it could potentially restart the pandemic.
While unproven and highly controversial, some researchers suspect that the Omicron variant may have evolved after the SARS-CoV-2 virus jumped to mice or other rodents (see Evidence for a mouse origin of the SARS-CoV-2 Omicron variant), and then spilled back into humans.
For more on this, see Maryn McKenna's Wired article Where Did Omicron Come From? Maybe Its First Host Was Mice.
All of which makes monitoring the spread and evolution of the SARS-CoV-2 virus in other, non-human, hosts essential if we are to avoid a repeat of the past few years.
Yesterday the Journal Nature published a lengthy, detailed, and at times technical report on the spread and evolution of SARS-CoV-2 in free-ranging deer across the United States.
This report is by researchers from the USDA/APHIS Wildlife Services, the CDC's NCIRD, and the Center for Influenza and Emerging Infectious Diseases at the University of Missouri, many of whom will be familiar to regular readers of this blog.
This is an excellent report, but due to its length I've only posted some limited excerpts. Follow the link to read it in its entirety. I'll have a postscript after the break.
Transmission of SARS-CoV-2 in free-ranging white-tailed deer in the United StatesAijing Feng, Sarah Bevins, Jeff Chandler, Thomas J. DeLiberto, Ria Ghai, Kristina Lantz,
Julianna Lenoch, Adam Retchless, Susan Shriner, Cynthia Y. Tang, Suxiang Sue Tong, Mia Torchetti, Anna Uehara & Xiu-Feng Wan
Nature Communications volume 14, Article number: 4078 (2023) Cite this article
Abstract
SARS-CoV-2 is a zoonotic virus with documented bi-directional transmission between people and animals. Transmission of SARS-CoV-2 from humans to free-ranging white-tailed deer (Odocoileus virginianus) poses a unique public health risk due to the potential for reservoir establishment where variants may persist and evolve.We collected 8,830 respiratory samples from free-ranging white-tailed deer across Washington, D.C. and 26 states in the United States between November 2021 and April 2022. We obtained 391 sequences and identified 34 Pango lineages including the Alpha, Gamma, Delta, and Omicron variants.Evolutionary analyses showed these white-tailed deer viruses originated from at least 109 independent spillovers from humans, which resulted in 39 cases of subsequent local deer-to-deer transmission and three cases of potential spillover from white-tailed deer back to humans. Viruses repeatedly adapted to white-tailed deer with recurring amino acid substitutions across spike and other proteins. Overall, our findings suggest that multiple SARS-CoV-2 lineages were introduced, became enzootic, and co-circulated in white-tailed deer.
(SNIP)
In summary, SARS-CoV-2 rapidly and repeatedly adapted to white-tailed deer with reoccurring and positively selected amino acid substitutions across the Spike protein (although not in the RBD), replicase, and other proteins.
(SNIP)
Efficient deer-to-deer transmission could facilitate establishment of white-tailed deer as a reservoir of SARS-CoV-2, presenting continuous risks for zoonotic transmission back to people and to other animals. The lineages with white-tailed deer-adaptive mutations may further increase the risk to humans. In addition, transmission of older SARS-CoV-2 viruses from white-tailed deer to people that are now uncommon in the human population may pose a greater risk as immunity to these lineages wane40, 41. Our findings highlight the potential public health implications of white-tailed deer-to-human transmission, but further studies with larger sample sizes are needed to fully understand the extent of transmission and the associated risks to human health.
Similar zoonotic transmission events have been documented in human seasonal H3N2 influenza A viruses, which spilled over from humans to pigs in the 1990s42, facilitating the generation of the 2009 pandemic virus through genetic reassortments with contemporary human, avian, and swine influenza viruses43. The swine H3N2 variant (H3N2v) virus remained antigenically more similar to human H3N2 precursor viruses in the 1990s but not contemporary seasonal H3N2 viruses in humans44. Since 2011, this H3N2v virus has frequently been transmitted back to humans and has caused 439 confirmed infections, particularly in those who were born after 2000 and had not yet been exposed to this virus subtype45.(SNIP)In summary, this study shows that SARS-CoV-2 was enzootic in white-tailed deer, and the viruses circulating in white-tailed deer stem from frequent and independent spillover events from humans with multiple genetic lineages co-circulating among white-tailed deer, including lineages observed in humans prior to and during our sampling period. Continued large-scale surveillance of white-tailed deer is necessary to understand the evolution and distribution of genetic variants in white-tailed deer, evaluate whether the white-tailed deer are a potential reservoir for SARS-CoV-2 viruses, and the role of white-tailed deer in ecology and natural history of SARS-CoV-2.
(Continue . . . )
J. Med. Virology: Potential Cross-Species Transmission Risks of Emerging Swine Enteric Coronavirus to Human Beings
Eurosurveillance: Cryptic SARS-CoV-2 Lineage Identified on Two Mink Farms In Poland
Nature: CoV Recombination Potential & The Need For the Development of Pan-CoV Vaccines
While the current lull in COVID cases is both welcomed and encouraging - with a diverse and growing array of mutable coronaviruses circulating in numerous host species - there are no guarantees how long it will last.