Non-polio Enteroviruses (NPEV's) - of which there are dozens identified - typically spread in the summer and early fall, and generally produce mild or even asymptomatic infections, mostly in children under the age of 10.
Symptomatic cases can range from a mild fever or a runny nose - to HFMD (Hand Foot Mouth Disease - a generally mild childhood disease characterized by blisters on the hand, feet, and mouth) - to more serious and even life threatening complications, including viral meningitis, viral encephalitis, myocarditis, pericarditis, and acute flaccid paralysis (AFP).
While most cases reported in the United States and in Europe have been from milder NPEVs (like Coxsackie A-16, A-10, or A-6), these viruses continue to evolve, and as they do they can acquire new traits, such as increased transmissibility or virulence.
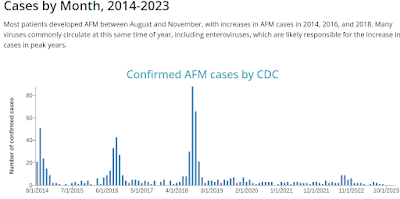
In 2014 a rarely reported, more virulent EV-D68 enterovirus emerged in the American Midwest, sparking a late summer respiratory outbreak in young children, which resulted in dozens of cases of Acute Flaccid Paralysis (see CDC HAN: Acute Neurologic Illness with Focal Limb Weakness of Unknown Etiology in Children).
This was followed by even larger outbreaks (in the U.S. and Europe) in 2016 and 2018 (see CDC Chart below). But the arrival of COVID, and the resultant pandemic precautions, has (at least temporarily) interrupted its bi-annual return.
In 2018 researchers identified changes in the EV-D68 virus that appear to have increased it's virulence (see mBio: Contemporary EV-D68 Strains Have Acquired The Ability To Infect Human Neuronal Cells); specifically differences in viral entry and replication between older (clade A) EV-D68 strains, and newer (clade B1, B2, and D1) strains, which appear to have emerged early in the 21st century.
A reminder that lest we get too comfortable, the status quo with any virus can always change over time.
- In 2009 China reported 1,155,525 HFMD cases, including 13,810 severe cases and 353 deaths. Among laboratory confirmed cases, EV71 was responsible for 41% of cases, 82% of severe cases, and 93% of the deaths (cite WHO HFMD Guide Pg.6).
- In 2012, we saw an outbreak of EV-71 in Cambodia that claimed the lives of dozens of children (see Updating The Cambodian EV71 Story).
- In 2013 in Australia: Acute Flaccid Paralysis & EV71, we looked at a report that described 5 cases of acute flaccid paralysis (AFP) in children who tested positive for the EV71 virus.
Like other RNA viruses we monitor, EV71 is constantly changing, evolving into new strains or lineages, and as a result we’ve seen repeated outbreaks of varying intensity over the years.
- During the late 1990s and early 2000s, genotypes C1, C2, B3, and B4 were most commonly reported as sparking outbreaks in Malaysia, Singapore, and Taiwan.
- By 2005 genotype C4 had replaced B4 in Taiwan, while in China C4 (which had split into 2 distinct lineages, C4a and C4b) caused major HFMD outbreaks in 2007–2009 (see Phylogenetic analysis of Enterovirus 71 circulating in Beijing, China from 2007 to 2009).
Although we've seen a small increase in the number of EV-71 cases in the United States and in Europe over the past decade, they remain relatively uncommon outside of Asia. But between the resumption of international travel - and new lineages of the virus emerging - there are legitimate concerns over its future spread.
Among the challenges of controlling EV71 outbreaks are:
- No currently available vaccine (outside of China)
- EV-71 is a moving viral target, with new strains evolving and emerging over time
- Many children can carry (and shed) the virus asymptomatically (see Incidence Rates of Enterovirus 71 Infections in Young Children during a Nationwide Epidemic in Taiwan, 2008–09)
- Patients may shed virus for month or longer (see Long persistence of EV71 specific nucleotides in respiratory and feces samples of the patients with Hand-Foot-Mouth Disease after recovery)
All of which brings us to a Dispatch, published late this week in the CDC's EID Journal, which describes a large outbreak of an emerging subgenogroup (B5) of the EV-71 Enterovirus in Vietnam over the past 12 months - its unusual presentation - and concerns over its potential spread beyond Vietnam.
Emerging Enterovirus A71 Subgenogroup B5 Causing Severe Hand, Foot, and Mouth Disease, Vietnam, 2023
Nguyen Van Vinh Chau, Tang Chi Thuong, Nguyen Thanh Hung, Nguyen Thi Thu Hong, Du Tuan Quy, Tran Ba Thien, Cao Minh Hiep, Ngo Ngoc Quang Minh, Truong Huu Khanh, Do Duong Kim Han, Truong Hoang Chau Truc, Nguyen Thi Han Ny, Le Kim Thanh, Lam Anh Nguyet, Cao Thu Thuy, Le Nguyen Truc Nhu, Pham Van Quang, Phung Nguyen The Nguyen, Phan Tu Qui, H. Rogier van Doorn, C. Louise Thwaites, Tran Tan Thanh, Nguyen Thanh Dung, Guy Thwaites, Nguyen To Anh, Le Nguyen Thanh Nhan, Le Van Tan, and for the SEACOVARIANTS1
Abstract
We report on a 2023 outbreak of severe hand, foot, and mouth disease in southern Vietnam caused by an emerging lineage of enterovirus A71 subgenogroup B5. Affected children were significantly older than those reported during previous outbreaks. The virus should be closely monitored to assess its potential for global dispersal.
Since 1997, large outbreaks of severe hand, foot, and mouth disease (HFMD) caused by diverse enterovirus A71 (EV-A71) subgenogroups (such as B4, B5, C4, and C5) have been reported in the Asia Pacific region (1), resulting in millions of hospitalizations and substantial numbers of deaths. Increased EV-A71 detection and associated neurologic disease have also been documented worldwide, including in the United States in more recent years (2).
During January 1–June 30, 2023, a total of 12,600 HFMD cases and 7 deaths were reported in Vietnam. Of those cases, 5,383 (42.7%) infections and all 7 deaths were recorded in June 2023. We investigated the epidemiologic and virologic features of this outbreak. The study was approved by the Institutional Review Board of CH1 and the Oxford University Tropical Research Ethics Committee. Written informed consent was obtained from a parent or guardian of each enrolled patient.
(SNIP)
Conclusions
We report that the 2023 outbreak of severe HFMD in Vietnam was caused by EV-A71 subgenogroups B5 and C1; B5 is dominant, and more older children were affected than during previous outbreaks. Phylogenetic analyses suggest that both B5 and C1 viruses were derived from new introductions of EV-A71 into Vietnam. In addition, the B5 viruses likely represent an emerging lineage because of a unique nonsynonymous amino acid substitution (S17G) in VP1 and because they form a distinct lineage within the global B5 phylogenetic tree. Further research is needed to clarify the origin and transmission network of this emerging lineage.
Underlying factors might cause the emergence of EV-A71 subgenogroups within a specific locality; the accumulation of a sufficient number of susceptible young children in the population and pathogen evolution might play critical roles (9,10). The changing epidemiology of respiratory pathogens as a consequence of COVID-19 has been documented (11), although EV-A71 is mainly transmitted by the oral-fecal route; thus, the effects of COVID-19 on EV-A71 transmission might be different from those of other respiratory viruses. However, the COVID-19 pandemic could have resulted in a large cohort of children who had greater susceptibility to EV-A71 infection, leading to a surge in infections among older children in the 2023 outbreak.
Virus immune evasion or altered virulence might also be substantial contributing factors in the outbreak (9,12). The amino acid residue 17 in VP1 does not form part of the identified EV-A71 immune epitopes (13), but mutations in the N terminus of VP1 might increase cell tropism, potentially contributing to EV-A71 pathogenesis. Collectively, because VP1 is the most immunogenic protein of EV-A71, the potential effects of the nonsynonymous S17G substitution on immune escape and virulence of EV-A71 subgenogroup B5 warrant further investigation.
Previous peaks of EV-A71 outbreaks in Vietnam occurred during September–November (3), coinciding with school reopening after the summer holiday (June–August). As of November 2023, the outbreak in Vietnam was still ongoing and had resulted in >100,000 infections and 23 deaths across the country.
The potential for severe EV-A71–associated HFMD outbreaks to spread to other parts of the world should be closely monitored.
Inactivated EV-A71 vaccines have been developed in China and Taiwan (14) but have only been used in China. Real-world data have shown that those vaccines substantially reduced EV-A71–associated disease transmission in China (15). Thus, using EV-A71 vaccines in other HFMD-endemic countries could have a similar effect. However, the extent to which EV-A71 vaccines might shape HFMD dynamics as a whole should be closely monitored. Because HFMD is transmitted through the oral-fecal route, good hygiene is critical to reduce EV-A71 transmission.
In conclusion, the 2023 outbreak of severe HFMD in Vietnam has mainly been caused by an emerging EV-A71 subgenogroup B5 lineage, and older children have been affected. Clinicians should recognize the diverse clinical manifestations of HFMD. Furthermore, enhanced EV-A71 surveillance is needed to inform the outbreak response in Vietnam and elsewhere, should the virus spread.
Dr. Chau is vice director of the Ho Chi Minh City Department of Health in Vietnam. His research interests focus on infectious diseases of public health importance in Vietnam, especially emerging infections.
Whether this iteration of EV-71 has the `right stuff' to spread beyond Asia remains to be seen, but this is a reminder that nature's laboratory is open 24/7, and it never stops tinkering.