#17,848
Fifteen years ago, in That Touch Of Mink Flu, we looked at a report out of Denmark where at least 11 mink farms in the Holstebro were reported to be infected with a variant of the human H3N2 virus. Also in 2009, an H3N2 Swine Influenza was detected in Canadian Mink.
- Three years earlier (2006) CIDRAP reported on a European mink found infected with an aggressive form of the H5 flu virus in the Blekinge region of southern Sweden near where H5N1 had been detected in wild birds.
- And in 1984 a large outbreak in Swedish mink farms was found to be due to the H10N4 avian virus.
SARS-CoV-2 mink-associated variant strain – Denmark
Disease Outbreak News: Update 3 December 2020
Since June 2020, Danish authorities have reported an extensive spread of SARS-CoV-2, the virus that causes COVID-19, on mink farms in Denmark. On 5 November, the Danish public health authorities reported the detection of a mink-associated SARS-CoV-2 variant with a combination of mutations not previously observed (referred to as “Cluster 5”) in 12 human cases in North Jutland, detected from August to September 2020.
Eurosurveillance: Cryptic SARS-CoV-2 Lineage Identified on Two Mink Farms In Poland
CDC: Investigating Possible Mink-To-Human Transmission Of SARS-CoV-2 In The United States
USDA APHIS Confirms SARS-CoV-2 in Farmed Mink in Utah
Although many mammalian species have shown a susceptibility to SARS-COV-2 (including deer, rodents, and household pets), mink - due to their physiology and the way they are farmed - pose unique risks.
Over the past 15 months - in addition to SARS-CoV-2 - we've seen a number of HPAI H5 avian flu infections in farmed mink (see here, here, and here), and last summer two well known UK virologists (T. Peacock & W. Barclay) penned the following opinion piece in the Journal PNAS.
Mink farming poses risks for future viral pandemicsThomas P. Peacock and Wendy S. Barclay
July 19, 2023
120 (30) e2303408120
https://doi.org/10.1073/pnas.2303408120
All of which brings us to a new review article - published today in the journal Viruses - which looks at the 4-year history of SARS-CoV-2 infection in farmed mink, and the potential for additional `mink-variants' to emerge in the future.
Due to its length, I've only posted the Abstract and Conclusions. Follow the link to read the review in its entirety. I'll have a postscript when you return.
by Mohammad Jawad Jahid, Andrew S. Bowman andJacqueline M. Nolting Department of Veterinary Preventive Medicine, College of Veterinary Medicine, The Ohio State University, Columbus, OH 43210, USA
Viruses 2024, 16(1), 81; https://doi.org/10.3390/v16010081 (registering DOI)
Published: 4 January 2024
ABSTRACT
Many studies have been conducted to explore outbreaks of SARS-CoV-2 in farmed mink and their intra-/inter-species spread and spillover to provide data to the scientific community, protecting human and animal health. Studies report anthropozoonotic introduction, which was initially documented in April 2020 in the Netherlands, and subsequent inter-/intra-species spread of SARS-CoV-2 in farmed mink, likely due to SARS-CoV-2 host tropism capable of establishing efficient interactions with host ACE2 and the mink hosts’ ability to enhance swift viral transmission due to their density, housing status, and occupational contacts.
Despite the rigorous prevention and control measures adopted, transmission of the virus within and between animal species was efficient, resulting in the development of mink-associated strains able to jump back and forth among the mink hosts and other animal/human contacts. Current knowledge recognizes the mink as a highly susceptible animal host harboring the virus with or without clinical manifestations, furthering infection transmission as a hidden animal reservoir. A One Health approach is, thus, recommended in SARS-CoV-2 surveillance and monitoring on mink farms and of their susceptible contact animals to identify and better understand these potential animal hosts.
(SNIP)
CONCLUSIONS
Mink are farmed in small cages, in high densities, and under conditions that can affect their immune system, facilitating the contraction and spread of disease. Upon introduction, a SARS-CoV-2 infection can spread swiftly on a mink farm, eventually affecting most, if not all, of the mink. Mink are the only known animal species found to spread SARS-CoV-2 to humans and other contact animals.
The first confirmed anthropozoonotic, human–mink, spread of SARS-CoV-2 was documented in the Netherlands, occurring in farmed mink. Following the virus’s introduction to farmed mink, there was quick intra-/inter-species transmission of SARS-CoV-2, which resulted in millions of mink being culled around the world. While infections in farmed mink were initially detected by observing severe respiratory symptoms and increased mortality, many infections were subclinical with no apparent clinical signs, making mink hidden animal reservoirs/sources for SARS-CoV-2. Massive and progressive mink–mink transmission of the virus led to the emergence of persistent infections in the mink population, resulting in the emergence of strains with mink-specific mutations resistant to neutralizing antibodies and thus challenging public health. Spillback events of these mink-specific strains to humans and subsequent human–human transmission leading to community spread of the virus have been reported.
In the effort to preclude further inter-/intra-species transmission of the virus and control outbreaks, strict biosecurity and biocontainment measures were adopted. Nonetheless, the measures were insufficient, as the virus continued to spread not only among mink but also from mink to other susceptible animals and humans. As observed in Denmark and many other affected places, the culling of farmed mink could have a role in reducing the incidence of epizootic and zoonotic events of the virus. Nonetheless, this causes a catastrophic economic loss for the mink industry [71].
Various studies have been conducted in the affected fur-producing countries to investigate the intra-/inter-species transmission dynamics of SARS-CoV-2 in farmed mink. Thinking of the triad of diseases in a broader array and in the case of SARS-CoV-2 with its zoonotic, anthropozoonotic, and epizootic capability, comprehensive studies were undertaken in most of the affected areas, including a broader range of domestic and wild animal contact species, human contacts (both on and around the farms in the community), and varied types of human, animal, and environmental samples. These studies have made a huge contribution to the current knowledge we have on SARS-CoV-2 in this potent animal species—farmed mink.
SARS-CoV-2 possesses a high mutation rate, broad host tropism, and high spillover potential. This necessitates the application of a comprehensive strategy to study its epidemiology and transmission dynamics to not only control its further spread and spillover within and between animal–human populations but also provide sufficient knowledge to the scientific and policymaking communities to better prepare, prevent, and respond to future epidemics/pandemics. Current knowledge thus recommends a One Health approach in the surveillance and monitoring of SARS-CoV-2 in farmed mink. A One Health approach will ensure multisectoral collaboration between planners and policymakers, scientific bodies, public health authorities, the fur industry, and public communities responding to this One Health threat.
While governments and health agencies have declared the COVID emergency to be over, SARS-CoV-2 continues to evolve, adapt, and expand its host range.
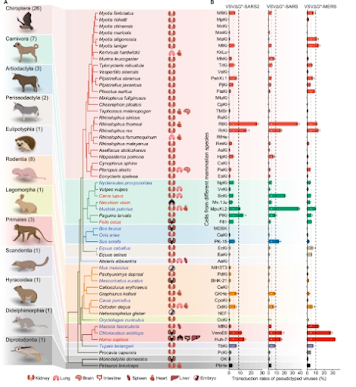
Today, numerous variants of SARS-CoV-2 circulate - alongside MERS-CoV, and other coronaviruses - in dozens of mammalian species (see Nature: Comparative Susceptibility of SARS-CoV-2, SARS-CoV, and MERS-CoV Across Mammals).
While that could arise from any susceptible animal source, the reality is farmed mink provide the virus with a near-perfect environment in which to evolve, and enough contact with humans to facilitate both zoonotic and reverse-zoonotic transmission.
The same holds true for novel (and seasonal) influenza, and we now know that it extends beyond mink to other farmed species, including foxes and raccoons (see Finland: Food Safety Authority Statement On H5N1 In Fur Farms).
Unfortunately, fur farming - like live poultry markets in Asia or the bush meat trade in Africa - are long held traditions, and despite their public health risks, getting people to move away from them has been a tough sell.
There are a lot of ways the next pandemic could emerge, many admittedly outside of our control. But given the stakes involved, where we can make changes - such as adopting the one-health approach to surveillance and disease control - we really should.