#18,475
With highly mutable influenza viruses the only constant is change, and HPAI H5 is no exception.
Over the past two decades we've watched as it has repeatedly reinvented itself, generating numerous subtypes (H5N1, H5N2, H5N5, H5N6, H5N8, etc.), dozens of subclades (2.2, 2.3.2.1a, 2.3.4.4b, etc.) and literally hundreds of genotypes (B3.13, D1.1, D1.2, etc.).
Most of these variants have fallen by the wayside, unable to compete with more `biologically fit' versions of the virus. But this winnowing process has also led to a far-more-capable group of viruses circulating today than 10 or 15 years ago.
Starting in 2020 we began to see a new subclade (2.3.4.4b) begin to spread globally, carried by a wider range of avian hosts (see DEFRA: The Unprecedented `Order Shift' In Wild Bird H5N1 Positives In Europe & The UK).
While we'd seen sporadic spillover of HPAI to non-human mammals (primarily cats), starting in 2021 we began to see reports of numerous spillovers into a much wider range of mammals (see chart below).Also of concern, in many cases these mammalian infections presented with severe neurological manifestations, to the point that many H5 infected animals were initially suspected to be rabid (see CDC EID Journal: Encephalitis and Death in Wild Mammals at An Animal Rehab Center From HPAI H5N8 - UK).
Over the past 9 months we've seen yet another seismic shift, with livestock (dairy cows, goats, alpacas, pigs, and even horses) - previously assumed immune - now susceptible to infection.
And since March of this year, we've seen an unprecedented surge in (mostly mild) human infections with the H5N1 virus in the United States. While the CDC only lists 58 confirmed cases, the real number (based on serological tests and anecdotal reports) suggests a much higher number.
This continued evolution, increased host range, and extrapulmonary spread in mammalian hosts is a genuine concern, as we really don't know what this H5 virus will do next.
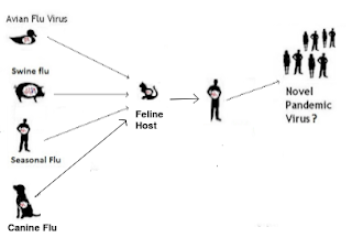
All of which brings us to a new research article, published yesterday in Emerging Microbes & Infections, that looks at a spillover of HPAI H5N1 earlier this year to a house full of domestic cats, all of which died, many with severe neurological symptoms.
The authors also document a number of potentially significant mutations in these cats, along with demonstrating their ability to serve as a potential `mixing vessel' for influenza.
This is a lengthy and highly detailed report, and it really deserves to be read in its entirety. I've posted the link, and some extended excerpts below. I'll have a postscript after the break.
Research Article
Marked Neurotropism and Potential Adaptation of H5N1 Clade 2.3.4.4.b Virus in Naturally Infected Domestic Cats
Shubhada K. Chothe, Surabhi Srinivas, Sougat Misra, Noel Chandan Nallipogu, Elizabeth Gilbride, Lindsey LaBella, Swastidipa Mukherjee, Christian H Gauthier, Heidi L. Pecoraro, Brett T. Webb, James M. Pipas, Santhamani Ramasamy & Suresh V. Kuchipudi
Article: 2440498 | Accepted author version posted online: 09 Dec 2024
Cite this article
https://doi.org/10.1080/22221751.2024.2440498
Abstract:
In April 2024, ten cats died in a rural South Dakota (SD) residence, showing respiratory and neurological symptoms. Necropsy and laboratory testing of two cats confirmed H5N1 clade 2.3.4.4b infection. The viral genome sequences are closely related to recent SD cattle H5N1 sequences.Cat H5N1 genomes had unique mutations, including T143A in haemagglutinin, known to affect infectivity and immune evasion, and two novel mutations in PA protein (F314L, L342Q) that may affect polymerase activity and virulence, suggesting potential virus adaptation. Dead cats showed systemic infection with lesions and viral antigens in multiple organs. Higher viral RNA and antigen in the brain indicated pronounced neurotropism.Lectin-histochemistry revealed widespread co-expression of sialic acid α-2,6 and α-2,3 receptors, suggesting cats could serve as mixing vessels for reassortment of avian and mammalian influenza viruses. No differences in clade 2.2 or 2.3.4.4b H5 pseudoviruses binding to cat lung/brain tissues indicated the neurotropism is unlikely mediated by receptor binding affinity.
Discussion
Increasing evidence suggests recent shifts in the patterns of mammalian infections with the HPAI H5N1 viruses worldwide, indicating ongoing adaptation to infect mammalian hosts [42] In addition, the host range of the HPAIV H5N1 virus has been expanding, with clade 2.3.4.4b spillovers now detected in various mammalian species. These include both domestic and wild carnivores, such as domestic cats [43], red foxes [44], multiple bear species [45], and seals [10] among others. This growing list of susceptible mammalian hosts highlights the virus's ability to cross species barriers, raising concerns about its potential impact on wildlife and domestic animal populations.
In this study, we report a natural H5N1 clade 2.3.4.4b virus infection resulting in the deaths of ten cats in rural South Dakota. The exact source of infection remains unclear; however, phylogenetic analysis of H5N1 sequences from two of the cats reveals a close genetic relationship to clade 2.3.4.4b strains previously detected in local cattle, suggesting a possible link. Additionally, the presence of bird feathers near the deceased cats indicates the likelihood that infection may have occurred through the consumption of virus-infected birds. However, because the disease typically requires several days to manifest post-ingestion, the exact timing of exposure is unclear. This evidence points toward a plausible cattle-to-bird-to-cat transmission pathway, supported by recent studies that identified H5N1 sequences across multiple species on affected farms, including dairy cows, wild birds, domestic cats, and raccoons [46].
Our study provides a significant new insight into the neurotropism of the H5N1 clade 2.3.4.4b virus in naturally infected domestic cats. There is a notable shift in the neurotropism of HPAI H5N1 viruses, particularly with the emergence of clade 2.3.4.4b in cats and wild carnivores like foxes. For example, in cats, experimental infection with the H5N1 Vietnam isolate (clade 2.2 - A/Vietnam/1194/04) showed primarily respiratory disease, with only one of three infected cats succumbing and no neurological symptoms[20]. Similarly, a natural H5N1 infection in Germany (A/swan/Germany/R65/06) in domestic cats displayed higher viral loads in the lungs than in the brain, with infection linked mainly to broncho-interstitial pneumonia [21]. Furthermore, studies on red foxes fed bird carcasses infected with clade 2.2 H5N1 also demonstrated limited clinical impact, with the foxes excreting the virus without developing severe disease. In contrast, recent H5N1 clade 2.3.4.4b infection in two cats from Texas [47] resulted in neurological signs and 50% mortality, likely due to ingestion of unpasteurized milk from infected cattle. Further, recent reports from Europe and the United States involving infection of red foxes with H5N1 clade 2.3.4.4b have shown a marked shift toward neurotropism [44]. These cases have documented viral adaptations that facilitate central nervous system involvement, with some infections exhibiting viral mutations indicative of enhanced neurotropism [46].
We identified several key mutations in the H5N1 sequence from infected cats that may suggest adaptation to cats. We observed a threonine-to-alanine mutation at residue 143 in HA (T143A). In A/Netherlands/219/2003 HA, this threonine (T143) forms a glycosylation motif (N-X-T/S) involving asparagine 141 (N141) around the receptor binding site (RBS), which is known to increase virus infectivity and resistance to neutralizing antibodies [48]. While the potential implications of the T143A mutation in clade 2.3.4.4.b are unclear, it could represent an adaptation mutation in cats that warrants further investigation. Notably, residue 143 in HA has been identified as a major mutation site in the RBS of H5 that contributes to viral escape from neutralizing antibodies among the different subclades, including 2.3.4.4b [49].
The N71S mutation in NA has not been previously reported in H5N1. While this mutation may not likely alter substrate specificity directly, it could potentially reduce efficiency on some substrates because phosphorylation or glycosylation of the serine residue might make the stalk of NA more rigid [50, 51]. The PA mutations (F314L and L342Q) are novel, and their functional implications are unknown. However, it is important to note that mutations in residues adjacent to these positions (343 and 347) in avian H5N1 influenza viruses have been shown to affect polymerase activity and virulence in mice [52]. Therefore, further investigation of these two PA mutations is critical to better understand their impact on the virus's polymerase activity and mammalian pathogenicity.
(SNIP)
In many rural households, as was the case with the infected cats in rural South Dakota reported in this study, cats are often housed outdoors, used for pest control, and considered family pets. This unique role exposes them to diverse environments and interactions, including terrestrial, aquatic, wild birds, and other livestock animals and humans. This exposure puts cats at a higher risk of encountering a broad spectrum of avian and mammalian influenza viruses. Notably, a recent study found that stray cats in the Netherlands were frequently exposed to HPAI H5, with a seropositivity rate of 11.8% among clinically healthy individuals [57]. The presence of asymptomatic infections in cats with H5N1 is a significant threat as these cats could serve as silent carriers, transmitting the virus to humans without showing any clinical signs of illness.
The continued exposure, viral circulation, and adaptation of the H5N1 virus in cats raise significant concerns for transmission and public health. Cats, common companion animals that frequently interact with humans and other species, could serve as a bridge for cross-species transmission of H5N1 viruses. Infected cats develop systemic infections and shed the virus through both respiratory and digestive tracts [58], potentially creating multiple routes of exposure to humans. Furthermore, the ability of the virus to persist and adapt in mammalian hosts heightens the risk of evolving into strains with increased transmissibility, posing an emerging zoonotic threat with profound public health implications. As H5N1 viruses continue to infect a wide range of avian and mammalian hosts, including an increasing number of human cases, there is an urgent need for coordinated One Health surveillance to monitor the spread of H5N1 among domestic and wild birds, animals, and humans.
While far from complete, the USDA lists > 2 dozen wildlife species of mammals affected by H5N1, including:
The state of South Dakota has reported 9 cats infected (see below), but this list only includes 2 of the 10 cats mentioned in this report. Most infected cats - like many other peridomestic species - are unlikely to be discovered or tested for the virus.While the CDC continues to rank the risk to general public from avian flu as low, they do provide very specific guidance to pet owners on how to limit their risk of infection from the virus (see What Causes Bird Flu in Pets and Other Animals).
And given the amount of HPAI virus being reported in wild birds, poultry, and livestock around the country, it is advice well worth heeding.


