Credit ECDC – Week 3
# 9642
While North America’s flu season is already well underway (and in some regions has already peaked), seasonal flu is getting a later - but no less strenuous start - in Europe this year. And as we’ve seen here in the United States, the predominant flu strain in Europe this season is a `drifted’ H3N2 virus, one which has reduced the effectiveness of this year’s vaccine.
This morning ECDC released an updated Rapid Risk Assessment and summary on this year’s flu season, and Director Dr. Marc Sprenger tweeted:
First a press release summary, with links to the Rapid Risk Assessment, and then I’ll be back with a few words on the CDC & ECDC’s strong recommendations for the use of antiviral medications.
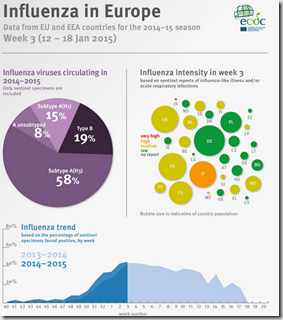
More severe influenza season to be expected in Europe
28 Jan 2015
Medium or high rates of influenza intensity are likely to be observed in the vast majority of EU and EEA countries, concludes ECDC annual risk assessment on influenza for the remainder of the season. The number of severe cases of influenza as well as fatal outcomes especially among older people and other risk groups can be expected to rise.
Strenuous start of this influenza season
- Influenza activity in Europe started in week 50/2014 without a particular geographic progression, affecting the Netherlands, Sweden and England first, and then followed by Iceland, Malta and Portugal.
- Children between 0 and four years of age have been the most affected age group according to primary healthcare data in almost all reporting countries, similarly as in other seasons.
- Influenza-like illness and acute respiratory infections have been increasing in adults and older people in almost all countries.
- Most of the first affected countries report greater pressure on primary healthcare services during this season compared to the peak activity in previous season.
- Among the countries reporting hospitalised influenza cases, 34 fatal outcomes were reported, two thirds of these in the elderly.
Drifted A(H3N2) viruses dominant
- Subtype A(H3N2) viruses, known to cause more severe disease, are dominant in almost all reporting European countries.
- Majority of A(H3N2) viruses analysed are antigenically distinct from the A(H3N2) virus included in the vaccine for this season.
- Reduced vaccine effectiveness is expected as a result of this mismatch between the vaccine and the circulating influenza strains.
ECDC Director, Dr Marc Sprenger, said:
“We face an influenza season that could be more severe and exert bigger pressure on health care systems than in the last few years. As each year, ECDC undertakes a risk assessment early in the season, combining a multitude of data sources and aiming to inform and strengthen EU and EEA countries in their response to the influenza epidemics.”
How to protect oneself and others from the flu
- Self-isolation when sick, hand-washing and good respiratory hygiene as well as cough etiquette remain simple yet effective measures to protect from catching or passing on influenza.
- A lower overall vaccine effectiveness due to the circulation of drifted A(H3N2) viruses is expected, however, the vaccine may still reduce complications and severe outcomes associated with this subtype of influenza viruses.
- Influenza vaccine offers good protection against the circulating A(H1N1)pdm09 viruses.
Antivirals particularly important this season
- Treatment and post-exposure prophylaxis with antivirals protects the elderly and people in other risk groups against severe influenza illness.
- The circulating viruses are susceptible to antiviral drugs oseltamivir and zanamivir.
Dr Marc Sprenger emphasizes:
“In a season dominated by a drifted A(H3N2) strain of influenza viruses, more severe illness can be expected especially among older people and those in medical risk groups. It is therefore paramount that physicians across Europe consider treatment and post-exposure prophylaxis with antivirals especially for these patients.”
The annual ECDC risk assessment of seasonal influenza aims to provide an early description of seasonal influenza in the first affected countries and to inform public health decisions to be taken to reduce the burden of seasonal influenza in 2015 in Europe.
Read full risk assessment of seasonal influenza in the EU/EEA countries, 2014-2015
More information:
Flu News Europe: weekly influenza updates
Seasonal influenza on ECDC website
Influenza maps and graphs
Follow us on Twitter: @ECDC_Flu
In Europe, even more so than in the US, antiviral drugs have been excoriated in the press; often referred to as an expensive scam on the part of the government, purportedly in cahoots with `Big Pharma’. In the past we’ve seen Tamiflu’s ® value questioned by Cochrane meta-studies, some prestigious medical journals, conspiracy theorists, pundits, but most often, the tabloid press.
Admittedly, it hasn’t helped that for many years Tamiflu’s maker - Roche Pharmaceuticals - has refused to release all of the testing data on their best selling antiviral drug, and we’ve seen some scare articles in the popular press suggesting adverse side effects to the drug.
With all of this baggage, you may be wondering why the ECDC, CDC , the UK’s PHE, and many other public health agencies continue to recommend the use of influenza antivirals for influenza.
Last April, in Revisiting Tamiflu Efficacy (Again), I wrote at some length on the BMJ – Cochrane Library review Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children – that examined a subset of the scientific literature and cast doubt on its effectiveness in treating influenza.
While I too lamented the lack of solid, well mounted Randomized controlled trials (RCTs) proving the effectiveness of Oseltamivir (particularly in high risk patients, or with novel flu strains), I listed a number observational studies that strongly support the effectiveness of Oseltamivir.
A few days later, the CDC issued their own response. I’ve posted the link and some excerpts below. Follow the link to read their rationale in its entirety.
CDC Recommendations for Influenza Antiviral Medications Remain Unchanged
April 10, 2014 -- CDC continues to recommend the use of the neuraminidase inhibitor antiviral drugs (oral oseltamivir and inhaled zanamivir) as an important adjunct to influenza vaccination in the treatment of influenza. CDC’s current influenza antiviral recommendations are available on the CDC website and are based on all available data, including the most recent Cochrane report, about the benefits of antiviral drugs in treating influenza.
Recommendations that were echoed a few months ago by Public Health England (see UK PHE: Revisiting Influenza Antiviral Recommendations), and that are supported by many studies I’ve written about previously, including:
Study: Antivirals Saved Lives Of Pregnant Women
BMJ: Efficacy of Oseltamivir In Mild H1N1
Study: The Benefits Of Antiviral Therapy During the 2009 Pandemic
The Lancet: Effectiveness Of NAI Antivirals In Reducing Mortality In Hospitalized H1N1pdm09 Cases
CID Journal: Under Utilization Of Antivirals For At Risk Flu Patients
For uncomplicated influenza in a healthy individual (essentially what the Cochrane studies looked at), antivirals probably offer little value.
But for severe influenza, or for people at risk of complications . . .
While not a cure, the preponderance of evidence shows that taking antivirals early can limit the severity and duration of symptoms – and for those patients – that could help keep them out of the hospital, and even prove life saving.