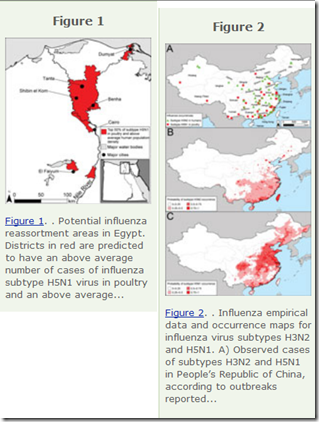
#14,590
In mid-March of 2013 - at a time when HPAI H5N1 was the world's primary avian flu concern - we examined a research article (see EID Journal: Predicting Hotspots for Influenza Virus Reassortment) that identifies 6 key geographic regions for avian flu reassortment (see graphic above).
The two biggest contenders? Coastal China, and the Nile Valley region of Egypt.Since timing is everything, just a little over 2 weeks later, China announced their first outbreak of LPAI H7N9, a virus that would evolve into both LPAI and HPAI strains (and two lineages) and spark 5 yearly epidemics (see below) before an effective vaccine was deployed in 2017.
 |
| H7N9 Epidemic Waves - June 14th - Credit FAO |
Eighteen months after the emergence of H7N9, Egypt saw the world's biggest HPAI H5 human outbreak (see EID Dispatch: Increased Number Of Human H5N1 Infection – Egypt, 2014-15), resulting in at least 165 infections, and 51 deaths.
Since then, Egypt has only reported sporadic human infections, but their surveillance and reporting has long been intermittent (see Revisiting Egypt’s Murky H5N1 Battle).But what we have seen has been a continual evolution of HPAI H5Nx viruses, with the introduction of HPAI H5N8 in 2016, and their reassortment with LPAI H9N2 (introduced in 2010). In the spring of 2019, a new, novel H5N2 appeared in Egypt (see Viruses: A Novel Reassortant H5N2 Virus In Egypt), which identified it as clade 2.3.4.4.
Eight months later, yet another novel H5N2 (a reassortment of H5N8 & H9N2) was described in the EID Journal (see Novel Reassortant HPAI A(H5N2) Virus in Broiler Chickens, Egypt).While Egypt has long maintained a poultry vaccination program, it has been plagued with vaccine failures and lapses in application (see Sci. Reports: Efficacy Of AI Vaccines Against The H5N8 Virus in Egypt) - which, unlike in China - has done little to curb the spread and evolution of avian flu viruses.
A few recent blogs include:
Emerg. Microbes & Inf.: Active Surveillance & Genetic Evolution of Avian Influenza Viruses in Egypt, 2016–2018To this growing list we can add a new study, published this week in Infection, Genetics and Evolution, that finds the pace of HPAI H5Nx evolution in Egypt continues to quicken, and warns that these viruses are picking up increased antiviral resistance as well.
Two Studies On The Recent Evolution Of HPAI H5 Viruses In The Middle East
J. Virology:Genetic Compatibility of Reassortants Between Avian H5N1 & H9N2 Influenza Viruses
Genotyping and reassortment analysis of highly pathogenic avian influenza viruses H5N8 and H5N2 from Egypt reveals successive annual replacement of genotypes
Kareem E.Hassan Noha Saad Hassanein H.Abozeid Salama Shany Magdy F.El-Kady Abdelsatar Arafa Azza A.A.EL-Sawah Florian Pfaff Hafez M.Hafez Martin Beer Timm Harder
https://doi.org/10.1016/j.meegid.2020.104375
Highlights
- Systematic reassortment analysis of all publicly available Egyptian whole genome sequences of HP H5N8, reassortant HP H5N2 and LP H9N2 viruses revealed presence of at least seven different genotypes of HPAI H5Nx viruses of clade 2.3.4.4b in Egypt.
- Heat mapping and tanglegram analyses suggested that several internal gene segments in both HP H5Nx and H9N2 viruses originated from avian influenza viruses circulating in wild bird species in Egypt.
- Amino acid substitutions in the Matrix (M2) and the Neuraminidase (NA) proteins of newly established sequences suggested presence of resistance against both Amantadine and Oseltamivir.
Abstract
Highly pathogenic (HP) H5N1, clade 2.2.1, and low pathogenic avian influenza (LPAI) H9N2 viruses, G1-B lineage, are endemic in poultry in Egypt and have co-circulated for almost a decade. Surprisingly, no inter-subtypic reassortment events have been reported from the field during that time.
After the introduction of HPAIV H5N8, clade 2.3.4.4b, in Egyptian poultry in 2016, suddenly HP H5N2 reassortants with H9N2 viruses emerged. The current analyses focussed on studying 32 duck flocks, 4 broiler chicken flocks, and 1 turkey flock, suffering from respiratory manifestations with moderate to high mortality reared in two Egyptian governorates during 2019. Real-time RT-PCR substantiated the presence of HP H5N8 in 21 of the 37 investigated flocks with mixed infection of H9N2 in two of them.
HP H5N1 was not detected. Full hemagglutinin (HA) sequencing of 10 samples with full-genome sequencing of three of them revealed presence of a single genotype. Very few substituting mutations in the HA protein were detected versus previous Egyptian HA sequences of that clade. Interestingly, amino acid substitutions in the Matrix (M2) and the Neuraminidase (NA) proteins associated with conferring both Amantadine and Oseltamivir resistance were present.
Systematic reassortment analysis of all publicly available Egyptian whole genome sequences of HP H5N8 (n = 23), reassortant HP H5N2 (n = 2) and LP H9N2 (n = 53) viruses revealed presence of at least seven different genotypes of HPAI H5Nx viruses of clade 2.3.4.4b in Egypt since 2016.
For H9N2 viruses, at least three genotypes were distinguishable.
Heat mapping and tanglegram analyses suggested that several internal gene segments in both HP H5Nx and H9N2 viruses originated from avian influenza viruses circulating in wild bird species in Egypt. Based on the limited set of whole genome sequences available, annual replacement patterns of HP H5Nx genotypes emerged and suggested selective advantages of certain genotypes since 2016.(Continue . . . )
While China and Southeast Asia have historically been world's primary hotspot for avian flu evolution, the Middle East - with its less-than-stellar infection control and a growing stable of circulating avian flu strains - has to be viewed as a serious contender.