#17,280
The $64 question with any emerging disease is whether it can successfully adapt to, replicate within, and spread between humans. When that happens - and assuming humans have little or no existing immunity - it can spread as an epidemic or even a pandemic.
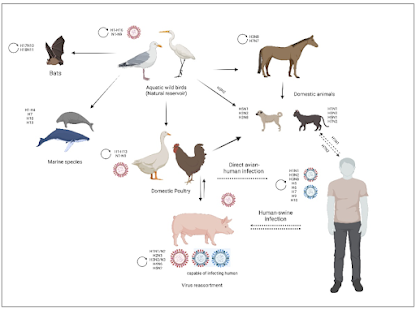
Influenza viruses - all of which appear to have originated from avian species - have managed to adapt to new hosts - including humans, horses, and swine - over thousands of years.
Twenty years ago, dogs and cats and bats were thought immune to influenza viruses, but in the early 2000's we saw equine H3N8 jump from horses to dogs for the first time (see EID Journal Influenza A Virus (H3N8) in Dogs with Respiratory Disease, Florida), while on the other side of the world, we were seeing big cats (tigers) infected, and dying from avian H5N1 in Thailand.
Since then, we've seen a number of flu strains infecting companion animals - and occasionally jumping to humans - including avian H7N2 at a New York City animal Shelter (see J Infect Dis: Serological Evidence Of H7N2 Infection Among Animal Shelter Workers, NYC 2016).
In 2012, we learned for the first time that there were bat-specific flu strains (see A New Flu Comes Up To Bat), and of a new class of flu viruses (Influenza D) that can infect cattle and swine (see EID journal’s Influenza D Virus in Cattle, France, 2011–2014 and EID Journal: Influenza D In Cattle & Swine – Italy).
Over the past decade we've seen repeated spillovers of avian influenza into marine mammals (see here, here, and here), and into farmed mink (see That Touch of Mink Flu (2023 Edition), and over the past year we've seen an unusual number avian flu infections in terrestrial mammals.
The current USDA report (see below) on H5N1 infected mammals (n=110) is almost certainly an undercount, as are reports from other countries.
And then there are the hundreds of human infections with avian flu (H5, H7, H9, H10, etc.), of which we probably only know of a small percentage (see WHO Update & Risk Assessment On Human H5N1 Infection - Ecuador).
So far, these avian flu viruses haven't figured out how to transmit efficiently from human-to-human, but they have demonstrated an ability to overcome those obstacles in a growing array of other mammalian species (pigs, dogs, horses, mink, etc.).
All of which brings us to a lengthy and detailed review of what we know about avian flu's expansion in other mammalian species, and what it may take for these viruses to become equally successful in human hosts.
Due to its length, I've only posted the abstract and some excerpts from the conclusion. Those thirsting for greater details will want to follow the link to read it in its entirety. But pack a lunch, because at 24 pages, you're going to be there a while.
Avian Influenza Virus Tropism in Humans
Umarqayum Abu Bakar ,Lina Amrani ,Farah Kamarulzaman ,Saiful Anuar Karsani ,Pouya Hassandarvish ,Jasmine Elanie Khairat *
Version 1 : Received: 4 February 2023 / Approved: 6 February 2023 / Online: 6 February 2023 (06:03:08 CET)
Bakar, U.A.; Amrani, L.; Kamarulzaman, F.; Karsani, S.A.; Hassandarvish, P.; Khairat, J.E. Avian Influenza Virus Tropism in Humans. Preprints 2023, 2023020081 (doi: 10.20944/preprints202302.0081.v1).
Abstract
A pandemic happens when a novel influenza A virus is able to infect and transmit efficiently to a new, distinct host species. Although the exact timing of pandemics is uncertain, it is known that both viral and host factors play a role in their emergence. Species-specific interactions between the virus and the host cell determine the virus tropism. These include binding and entering cells, replicating the viral RNA genome within the host cell nucleus, assembling, maturing, and releasing the virus to neighbouring cells, tissues, or organs before transmitting it between individuals.Influenza has a vast and antigenically varied reservoir. In wild aquatic birds, the infection is typically asymptomatic. Avian influenza virus (AIV) can cross into new species, and occasionally, it can acquire the ability to transmit from human to human. A pandemic might occur if a new influenza virus acquires enough adaptive mutations to maintain transmission between people.This review highlights the key determinants AIV must achieve to initiate a human pandemic and describes how AIV mutates to establish tropism and stable human adaptation. Understanding the tropism of AIV may be crucial in preventing virus transmission in humans and may help design vaccines, antivirals and therapeutic agents against the virus.
(SNIP)
6. Conclusions
Many subtypes of AIV have been confirmed to infect humans, including H3N8, H5N1, H5N6, H5N8, H6N1, H7N1, H7N2, H7N3, H7N4, H7N7, H7N9, H9N2, H10N3, H10N7 and H10N8 [170,171]. Among these were the highly virulent subtypes H5N1 and H7N9, which caused high mortality in humans. Since its first appearance, H5N1 has been linked to more than 800 cases and 400 deaths globally, whereas H7N9 has been responsible for more than 1500 human cases and 600 deaths worldwide [172].
Most AIV subtype infections were caused mainly by exposure to infected poultry, suggesting that human-to-human transmission of AIV is still ineffective. However, given what we currently know about the rapid adaptation of AIV, efficient human-to-human transmission is possible, and it is just a matter of time before it happens.
Therefore, we have highlighted the key determinants for AIV tropism in humans according to the most frequently reported virus and host factors. Based on that, we suggest the minimal requirement for efficient AIV transmission between humans that could initiate new pandemics:
(i) adaptation of receptor binding preference of HA to α2,6 SA during attachment;
(ii) optimum HA stability during membrane fusion;
(iii) the improvement of efficiency during the import of vRNP into the nucleus
(iv) the increase of polymerase activity and flexibility during transcription and replication;
(v) the balance in M2/M1 expression after M segment splicing;
(vi) efficient assembly of the RdRP complex;
(vii) co-transport of the M1-GNB1 complex to the budding site;
(viii) a functional balance between HA affinity and NA enzymatic activity with SA.
It is necessary to be prepared for the worst since we cannot predict what types of mutations may occur in the future that could potentially cause pandemics. Understanding the mechanisms that underlie adaptive changes and the circumstances in which they can be acquired will enhance our ability to assess the public health risks posed by AIV and predict the source of future pandemics. It will also reveal intricate details of virus-host interactions that can lead to the development of novel vaccines, antiviral agents and effective therapeutic strategies.
