#17,267
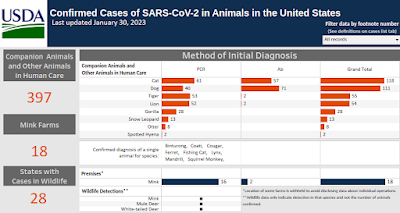
Although it is now an exquisitely human-adapted virus, SARS-CoV-2 likely began in a bat and jumped to humans in 2019. Its host range, however, now extends far beyond humans and bats, with a growing number of mammalian species - including cats, dogs, mink, deer, and monkeys - proving to be susceptible to the virus.
While most human infections come from exposure to other humans, we have seen rare examples (see below) of mink-to-human, cat-to-human, and even suspected deer-to-human transmission, sometimes with `mutated' COVID viruses carrying unique host adaptations.
EID Journal: Suspected Cat-to-Human Transmission of SARS-CoV-2 - Thailand
Nature: Divergent SARS-CoV-2 Variant Emerges in White-tailed Deer with Deer-to-Human Transmission (Revisited)
ECDC Rapid Risk Assessment: Detection of New SARS-CoV-2 Variants Related to Mink
These events raise concerns that the virus could follow divergent evolutionary paths in non-human hosts, producing new, potentially dangerous variants that could eventually `spillback' into humans. Ten months ago the WHO/FAO/OIE issued a Joint Statement On Monitoring SARS-CoV-2 In Wildlife & Preventing Formation of Reservoirs.
While this sort of extended zoonotic transmission has only rarely been reported, it is likely that some (perhaps many) cases go undetected.
All of which brings us to a preprint - published yesterday on the MedRxiv server - that details the probable transmission of COVID from an African Lion at a zoo in Indiana to several zoo employees.
I've only posted the Abstract and some excerpts from the report, so follow the link to read it in its entirety.
Probable transmission of SARS-CoV-2 from an African lion to zoo employees
Audrey A Siegrist, Kira L Richardson, Ria R Ghai, Brian Pope, Jamie Yeadon, Betsy Culp, Casey Barton Behravesh, Lixia Liu, Jennifer A Brown, Leslie Boyerdoi: https://doi.org/10.1101/2023.01.29.23285159Animal to human transmission of SARS-CoV-2 has not previously been reported in a zoo setting. A vaccinated African lion with physical limitations requiring hand feeding tested positive for SARS-CoV-2 after development of respiratory signs. Zoo employees were screened, monitored prospectively for development of symptoms, then re-screened as indicated, with confirmation by RT-PCR and whole-genome virus sequencing when possible. Trace-back investigation narrowed the source of infection to one of five people.
Three exposed employees subsequently developed symptoms, two with viral genomes identical to the lion's. Forward contact tracing investigation confirmed probable lion-to-human transmission. Close contact with large cats is a risk factor for bidirectional zoonotic SARS-CoV-2 transmission that should be considered when occupational health and biosecurity practices at zoos are designed and implemented. SARS-CoV-2 rapid testing and detection methods in big cats and other susceptible animals should be developed and validated to facilitate timely implementation of One Health investigations.
(SNIP)
Discussion
This multispecies cluster of SARS-CoV-2 included three confirmed cases (two human, one felid) and one probable case (human). The identical genomic sequences detected in samples from the confirmed cases demonstrate that the infections were acquired in a common setting. Given that the zoo was closed to visitors, the source of infection for the sentinel case was almost certainly one of 6 asymptomatic employees who tested negative on the day of the lion’s diagnosis and who subsequently reported no signs of illness. Among these 6, Employee Z5, the only person who had cranial contact within 5 days before the lion’s onset of illness and who did not later develop symptomatic COVID-19, was the most likely source of the lion’s infection
(SNIP)
Our investigation strongly suggests that lion-to-human transmission took place in at least one, and up to three, instances.
This is important for at least two reasons. First, animal-to-person transmission of SARS-CoV-2 is an occupational health risk for veterinary and animal care staff that interact closely with susceptible animals. Second, transmission occurred despite an up-todate SARS-CoV-2 vaccination history in every individual involved, including the person(s) that likely transmitted the virus to the lion, the lion, and the person(s) that likely became infected from the lion.
While SARS-CoV-2 transmission from vaccinated people to vaccinated zoo animals has been documented previously23, our results show that transmission can also occur from vaccinated zoo animals to vaccinated people. While many human vaccine efficacy studies indicate reductions in disease severity of COVID-19 following vaccination24,25, further research is needed to determine if the same is true of zoonotic SARS-CoV-2 transmission among vaccinated people and animals.
(Continue . . . )
Although this `mink variant' was quickly overtaken by the Alpha variant - and no longer circulates - over the summer of 2021 we learned from an SSI Study: Denmark's Cluster-5 mink Variant Had Increased Antibody Resistance.
This served as a `proof of concept' that - if allowed to spread in a non-human species - SARS-CoV-2 could evolve into something `new' and potentially more dangerous, and spill back into the human population with unpredictable results.
Which is why we follow reports such as the one presented above with considerable interest.