#17,401
While the COVID pandemic appears to have - at least temporarily - stabilized with the Omicron sublineage, and many are hoping the end of the emergency is at hand, there is a wildcard that keeps a lot of researchers up at night.
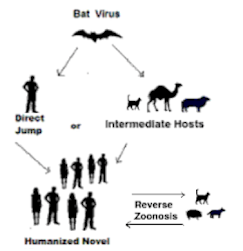
Although that could well happen through a recombination event in humans (see Nature: CoV Recombination Potential & The Need For the Development of Pan-CoV Vaccines), another plausible route would be from a COVID virus that successfully embarked upon a divergent evolutionary path in a non-human host.The possibility that a radically different variant could emerge for which we have little or no immunity, and kick off another pandemic wave.
Much of the data we have on non-human infection with SARS-CoV-2 comes from companion animals, and/or captive or farmed animals.
Most pets in households affected by COVID are never tested (unless they are symptomatic), but we have seen hundreds of companion animals in the United States alone which have tested positive for SARS-CoV-2 infection (see USDA chart below).
How often SARS-CoV-2 infects animals in the wild is largely unknown, but the spillover of the virus into other species is increasingly viewed as a serious threat (see WHO/FAO/OIE Joint Statement On Monitoring SARS-CoV-2 In Wildlife & Preventing Formation of Reservoirs).
- Last November, a preprint (see Wildlife Exposure to SARS-CoV-2 Across a Human Use Gradient) found evidence of SARS-CoV-2 infection across a wide variety of small peridomestic mammals (e.g. possums, skunks, squirrels, etc.) in Virginia
- Another study (see SARS-CoV-2 Exposure in Norwegian rats (Rattus norvegicus) from New York City) found evidence of spillover to NYC rats.
- And we've seen repeated reports of SARS-CoV-2 spreading (and evolving) in North American deer (see PNAS: White-Tailed Deer as a Wildlife Reservoir for Nearly Extinct SARS-CoV-2 Variants
- And in farmed mink (see Denmark SSI: Low to Moderate Risk of Human Infection With Bird Flu From Mink).
All of which serves as background to a research letter, published yesterday in the CDC's EID Journal, which reports the testing of companion animals in South Korean households where at least one human occupant was infected with COVID.
As with the USDA data above, cats outnumber dogs in detections of the virus by about 6 to 4.
This report suggests (based on limited data) that the incidence of COVID infection of household pets may be much higher than previously suspected.
I've have a brief postscript after the break.
Volume 29, Number 5—May 2023
Research Letter
Human-to-Animal Transmission of SARS-CoV-2, South Korea, 2021
Jinsun Bae1, Changseek Ro1, Yunhee Kang, Eulhae Ga, Woonsung Na, and Daesub Song

Abstract
To investigate SARS-CoV-2 transmission from humans to animals in Seoul, South Korea, we submitted samples from companion animals owned by persons with confirmed COVID-19. Real-time PCR indicated higher SARS-CoV-2 viral infection rates for dogs and cats than previously reported from the United States and Europe. Host-specific adaptations could introduce mutant SARS-CoV-2 to humans.
The risk for zoonoses (animal-to-human transmission) is increasing as human and wildlife habitats overlap with more human and animal migration and industrial food animals worldwide. Reverse zoonosis (human-to-animal transmission) also occurs (1–5). The main concern about reverse zoonosis is the possibility that an animal can act as a carrier and reinfect a person.
According to South Korea government health policy, every confirmed human case of COVID-19 is reported to the regional public health center, and epidemiologic investigations began in February 2021. To determine possible human-to-animal transmission of SARS-CoV-2, we surveyed SARS-CoV-2 results for companion animals (dogs and cats) owned by persons with confirmed COVID-19 who were living in Seoul during February–November 2021. We assessed only companion animals for which owners were confirmed and for which owners had requested testing. A total of 375 companion animals (271 dogs and 104 cats) were tested for SARS-CoV-2 by real-time PCR.
When a companion animal exhibits suspected clinical signs and its owner requests a test, the Seoul city animal specimen collection team is dispatched to collect samples. For this study, the veterinarian managing the protection facility collected samples from companion animals whose owners had been confirmed to have COVID-19 and transferred the animals to separate protection facilities. Sampling was conducted according to guidelines of the World Organisation for Animal Health (6). Samples were collected by swabbing 3 locations on the animals: the oropharynx, nasal cavity, and rectum. The samples were transferred to individual virus transport media (1 mL), packaged in 3-layer biosafety packaging containers, and transported to the testing facility while refrigeration was maintained.
The COVID-19 diagnosis was established by using the real-time PCR method recommended by the World Health Organization to determine the presence or absence of SARS-CovV-2 virus antigens (7). Among the amplification genes, both RdRp (RNA-dependent RNA polymerase) and E genes were detected, indicating SARS-CoV-2 positivity; cycle threshold for each was <38. When PCR was positive for samples from >1 of the 3 sampling sites, the animal was determined to have a positive result.
Using SPSS Statistics 24 (IBM, https://www.ibm.com), we cross-tabulated and statistically analyzed the COVID-19 infection rate for companion dogs and cats owned by persons with confirmed cases of COVID-19. We found that 102 (27.2%) of 375 animals examined had positive results for SARS-CoV-2 infection: 65 (24%) dogs and 37 (35.6%) cats (Table). When we compared the positivity rates for the 2 species, we found that the positivity rate for cats was significantly higher than that for dogs (p<0.024).
We also investigated the rate of positivity detection according to sampling site. The positivity rate was higher for samples collected from the oropharynx (72.41%) and nasal cavity (84.85%) of dogs and from the oropharynx (83.33%) and nasal cavity (75.0%) of cats than from the rectum from either species (30.3% for dogs and 51.43% for cats).
This study reveals SARS-CoV-2 positivity rates of 24.0% for dogs and 35.6% for cats in South Korea, higher than rates previously reported from studies of dogs and cats. Although the animals in our study were already known to have been exposed to SARS-CoV-2 because their owners were confirmed to have COVID-19, the rate of positivity is high compared with rates determined in previous studies of animals with SARS-CoV-2–positive owners (8,9). This finding emphasizes the value and necessity of managing infectious diseases in companion animals as well as in humans because the risk for reverse zoonoses increases when companion animals are in prolonged and close contact with their owners.
Our study was limited by having been conducted with animals consigned to the protection facilities of the Seoul City Government and those whose tests were requested by their owners because of the animals’ clinical signs. Owner bias might have affected the population in this setting.
Our study could provide epidemiologically meaningful data for public health. As SARS-CoV-2 spreads as a pandemic, reverse zoonotic infections will continue, and viruses will mutate to adapt to the new host. For companion animals living near humans, continuous epidemiologic investigations and monitoring will be needed.
Dr. Bae is a leader of the veterinary public health section of the Seoul Metropolitan government. Her research interests include the epidemiology of zoonoses, infectious disease prevention policies and administrative affairs such as quarantine.
Eighteen months ago we looked at a perspective article, published in China's CCDC Weekly by two well-known Chinese scientists - George F. Gao and Liang Wang - on the continual spread of SARS-CoV-2 from humans to other animal hosts, and the impacts that could have going forward (see Perspectives: COVID-19 Expands Its Territories from Humans to Animals).
The authors warned that the potential for seeing new and dangerous variants emerge - particularly in wild and domesticated animals - was very high.
Three months ago we looked at another cautionary correspondence from researchers from Guangdong, China and Toronto, Canada published in The Lancet Microbe in late December of 2022 (see Ecology of SARS-CoV-2 in the Post-Pandemic Era).
The authors wrote:
SARS-CoV-2 transmission and reverse transmission from humans to animals that are in close contact with humans, such as zoo animals (pumas, tigers, lions, and gorillas), fur-bearing animals (minks and ferrets), and pets (cats and dogs) has been widely reported.2, 3
The pet population is very large in industrialised nations, where a high proportion of households have pets. For example, 25·4% of households in the USA have cats (data from the American Veterinary Medical Association).
Considering the intimate contact between pets and zoo animals with humans, especially the large number of pets, the circulation of SARS-CoV-2 in these animals should not be neglected (appendix).
While we appear to be moving ever closer to the end of our current COVID emergency, a new zoonotic spillover of a mutated variant could present the world with an enormous setback.