#17,723
After a marked increase in fatal heart attacks in New York City during the first pandemic wave (see JAMA Investigation) - and autopsy reports on 39 COVID cases - cardiologists began to openly discuss potential long-term impact of COVID infection (see Coronavirus Disease 2019 (COVID-19) and the Heart—Is Heart Failure the Next Chapter? Clyde W. Yancy, MD, MSc1,2; Gregg C. Fonarow, MD3,4)
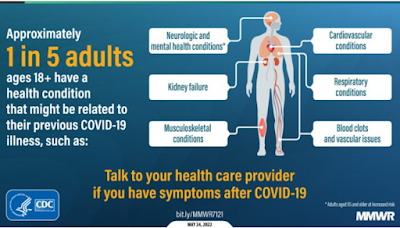
At the same time, additional reports emerged describing neurological manifestations in COVID patients, including:
- The Lancet: Yet Another Study On Neurological Manifestations In Severe COVID-19 Patients
- The emerging spectrum of COVID-19 neurology.
- In August, in J. Neurology: COVID-19 As A Potential Risk Factor For Chronic Neurological. Disorders, we revisited the growing concerns over the long-term neurological impacts of COVID-19 infection.
- in September 2020 (see Parkinsonism as a Third Wave of the COVID-19 Pandemic?) we looked at a Review Article that examined the parallels between COVID-19 and epidemics of the past, and the potential for seeing a new wave of neurological disorders.
- Another warning came in Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms by Emily A. Troyer, Jordan N. Kohn, and Suzi Hong).
All of this is terribly inconvenient for a world that desperately wants to `move on' from COVID, and treat the virus as a relatively benign seasonal virus.
While there is growing evidence suggesting we may be facing an as-yet unquantified health burden from Long-COVID, the scope, impact, and etiology of these sequelae are only partially understood.
Studies, like today's research article in the EID Journal, will hopefully fill in some of those gaps. The caveat being that animals studies don't always translate perfectly to humans.
Due to its length, I've only posted some brief excerpts. Follow the link to read it in its entirety. I'll have a postscript when you return.
Research
Neurologic Effects of SARS-CoV-2 Transmitted among Dogs
Dong-Hwi Kim1, Da-Yoon Kim1, Kyu-Sung Kim1, Sang-Hoon Han, Hyeon-Jeong Go, Jae-Hyeong Kim, Kyu-Beom Lim, Dong-Hun Lee, Joong-Bok Lee, Seung-Yong Park, Chang-Seon Song, Sang-Won Lee, Yang-Kyu Choi, Yeun-Kyung Shin, Oh-Kyu Kwon, Do-Geun Kim, and In-Soo Choi
Abstract
SARS-CoV-2 induces illness and death in humans by causing systemic infections. Evidence suggests that SARS-CoV-2 can induce brain pathology in humans and other hosts. In this study, we used a canine transmission model to examine histopathologic changes in the brains of dogs infected with SARS-CoV-2. We observed substantial brain pathology in SARS-CoV-2–infected dogs, particularly involving blood–brain barrier damage resembling small vessel disease, including changes in tight junction proteins, reduced laminin levels, and decreased pericyte coverage.
Furthermore, we detected phosphorylated tau, a marker of neurodegenerative disease, indicating a potential link between SARS-CoV-2–associated small vessel disease and neurodegeneration.Our findings of degenerative changes in the dog brain during SARS-CoV-2 infection emphasize the potential for transmission to other hosts and induction of similar signs and symptoms. The dynamic brain changes in dogs highlight that even asymptomatic individuals infected with SARS-CoV-2 may develop neuropathologic changes in the brain.
Furthermore, cortical thickness is reduced in SARS-CoV-2–infected patients, suggesting that SARS-CoV-2 can induce pathologic changes in the brain, which may be linked to the functional deficits noted in those patients. Considering the number of patients infected with SARS-CoV-2, the neurologic signs can lead to a potential wave of neurodegenerative diseases, which could pose an immense burden on society.
The etiology of SARS-CoV-2–induced neuropathologic changes is still elusive. However, clinical and experimental reports suggest that vascular damage and the resultant immune responses in the brain may be a major factor (13–16). Magnetic resonance imaging has detected white matter hyperintensities in SARS-CoV-2–infected patients, indicating damage to the blood–brain barrier (BBB) in this region and that potentially demyelinating pathologic changes can be induced (13).
Other studies have revealed signs of neuroinflammatory responses, including activation of microglial cells and astrocytes (14,15). Moreover, damage to the brain vasculature and defects in the coagulation system have been demonstrated (16). The characteristic pathologies observed in human patients (e.g., vascular damage, demyelination, and neuroinflammatory responses) have also been observed in humanized mouse models.
We used a canine transmission model to investigate the susceptibility of dogs to SARS-CoV-2, specifically the Delta variant. The dogs were housed in a Biosafety Level 3 animal facility at Konkuk University Laboratory, Seoul, South Korea, where temperature, humidity, and light were carefully controlled. The study was approved by the Animal Research Center under the supervision of the Institutional Animal Care and Use Committee (accreditation no. KU22065) and the Institutional Biosafety Committee (accreditation no. KUIBC-2022-06) at Konkuk University. The absence of SARS-CoV-2 RNA and SARS-CoV-2 antibodies in dog serum was confirmed.
(SNIP)
Discussion
Overall, our study demonstrates solid experimental evidence that SARS-CoV-2 can infect dogs and be transmitted to others by direct contact, producing pathologic brain changes even without prominent signs. Pathologic changes in the lung and brain were observed in dogs of both groups, providing additional evidence of virus transmission. Of note, SARS-CoV-2 infection has been reported to cause long-term pathologic effects even after the virus is cleared from the main organs of the body (17).
Our study provides evidence that SARS-CoV-2 infection can damage the brain as well as the lungs in dogs at early and later stages of infection, suggesting a high potential for a long-lasting COVID-19–like syndrome to develop in affected dogs.
(SNIP)
Among the merits of our study in terms of translational research of SARS-CoV-2–induced neuropathologic changes, we compared 2 infection routes: direct intranasal infection and horizontal transmission models that can mimic more natural infection routes. With that comparison, we determined that neuropathologic changes can be induced via both exposure routes, providing valuable information that owners of companion animals potentially face SARS-CoV-2–associated neurologic disorders.
Second, we studied dogs, which are a more advanced species than rodents, to provide neuropathologic data that are closer to data for humans and more relevant. Moreover, our data suggest that neuropathologic changes can be induced in dogs. Last, we found that the neuroinflammatory responses were more prominently observed in the white matter area than the gray matter area, suggesting that the neuroinflammatory responses induced by SARS-CoV-2 differ by brain region. Overall, these data can be used as translational research data to interpret the potential neuropathologic changes that may be observed in humans.
(SNIP)
D.-H. Kim is a PhD candidate at Konkuk University in Seoul. His primary research interests include diagnostics, vaccine development, and antiviral therapeutics, with a particular emphasis on zoonotic viruses.
After all, there is historical precedence.
While some scientists believed this disease - called Encephalitis Lethargica (EL) - was a rare sequelae of the 1918 pandemic virus, the exact cause remains unknown. A post-viral infection, however, remains a likely culprit (see Evidence for an enterovirus as the cause of encephalitis lethargica).
Regardless, millions died, or were institutionalized.
Throughout history, there have been reports of similar outbreaks, resulting in severe neurological disease, including febris comatosa which sparked a severe epidemic in London between 1673 and 1675, and in 1890 in Italy, in the wake of the 1889–1890 influenza (?) pandemic, a severe wave of somnolent illnesses (nicknamed the "Nona") appeared.
While hopefully we aren't in for a repeat with COVID, we probably won't have a good handle on the long-term impacts of COVID infection for years to come.
Which is why it still makes sense to avoid COVID infection whenever possible. At least, until we know more.