Credit ECDC Tuberculosis monitoring and surveillance 2015
# 9837
Although we tend to focus mostly on new and emerging health threats, old scourges like Tuberculosis continue to impact global health, and claims over a million lives each year.
Each March, we observe World TB day on the anniversary when Dr. Robert Koch announced his discovery of Mycobacterium tuberculosis in 1882.
First some quick facts about TB from the World Health Organization, followed by a press release from the ECDC on their annual TB surveillance and progress report, and a joint statement between the ECDC and WHO.
Tuberculosis
Fact sheet N°104
Reviewed March 2015
Key facts
- Tuberculosis (TB) is second only to HIV/AIDS as the greatest killer worldwide due to a single infectious agent.
- In 2013, 9 million people fell ill with TB and 1.5 million died from the disease.
- Over 95% of TB deaths occur in low- and middle-income countries, and it is among the top 5 causes of death for women aged 15 to 44.
- In 2013, an estimated 550 000 children became ill with TB and 80 000 HIV-negative children died of TB.
- TB is a leading killer of HIV-positive people causing one fourth of all HIV-related deaths.
- Globally in 2013, an estimated 480 000 people developed multidrug resistant TB (MDR-TB).
- The estimated number of people falling ill with TB each year is declining, although very slowly, which means that the world is on track to achieve the Millennium Development Goal to reverse the spread of TB by 2015.
- The TB death rate dropped 45% between 1990 and 2013.
- An estimated 37 million lives were saved through TB diagnosis and treatment between 2000 and 2013.
This from the ECDC.
Tuberculosis cases down by 6% in 2013 – pace too slow to reach elimination this century
17 Mar 2015
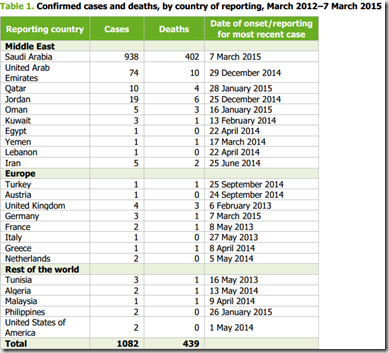
In 2013, 64 844 tuberculosis cases were reported by 30 EU/EEA Member States, according to new data published today by the European Centre for Disease Prevention and Control and the WHO Regional Office for Europe. The notification rate of 12.7 per 100 000 population observed in 2013 constitutes a 6% decrease compared to the previous year, when some 68 000 cases were notified.
The overall downward trend within the European Union and European Economic Area (EU/EEA) is influenced by a marked decline of TB in high-incidence countries such as Romania, which accounts for 26% of all reported cases in the EU/EEA, whereas in some low-incidence countries like Denmark, Norway and Sweden, notification rates are actually going up.
“Our data show a Europe in need of tailored interventions which target each country’s settings”, says ECDC Director Marc Sprenger ahead of World TB Day. Despite historically low numbers and a significant decline over the last ten years, the EU/EEA countries are not all progressing in the same way and face specific challenges in their TB control efforts. In most low-incidence countries, rates are stable or going down only very slowly and the majority of patients are of foreign-origin. Countries with high incidence overall face higher rates of re-infection and relapses and report many more multidrug-resistant (MDR TB) cases.
According to the new data, only 4% of TB cases tested for drug-resistance are MDR TB. However, treatment success rates for these cases are very low and have remained unchanged over the past 10 years.
TB elimination still too far away
“At the current pace of an annual 6% decline, the EU/EEA will only be free of tuberculosis in the next century. In order to achieve elimination by 2050 for example, we would have to cut down cases at least twice as fast”, warns Sprenger. In order to achieve TB elimination, current tools and interventions like early diagnosis, correct treatment and contact tracing have to be used more efficiently. In a next step, the currently available tools have to be complemented by new and more effective ones, including better diagnostic tools, shorter treatment and an effective vaccine.ECDC working with countries
The goal of TB prevention and control will be one of the main topics of the first Ministerial Conference on TB and MDR TB, to be held in Riga on 30-31 March under the Latvian EU Council Presidency, and where ECDC will be participating. On 1-2 April, a technical meeting organized by ECDC and the Centre for Disease Prevention and Control of Latvia will discuss how to address TB and MDR TB in high-priority countries.Tuberculosis monitoring and surveillance 2015
Credit ECDC – Warning LARGE PDF
Every day, 1 000 people get sick with tuberculosis in the European Region
Stockholm/Copenhagen 17/3/2015An estimated 360 000 Europeans developed tuberculosis (TB) in 2013 – 1 000 people on a daily basis. According to new data published today by the European Centre for Disease Prevention and Control and the WHO Regional Office for Europe, the number of TB cases dropped by about 6% compared to 2012, continuing a sustained decline over the last decade across the Region. But rates of multidrug-resistant (MDR) TB remain at very high levels, particularly in the so-called 18 high priority countries which see 85% of all new TB cases in the Region. These countries also account for most of the 38 000 TB-related deaths in 2013.