#18,383
Yesterday the USDA published the following statement indicating that they plan to expand testing for H5N1 in dairy cattle, although they provide few details, and it isn't at all clear to what extent (if at all) testing will become mandatory.
Given the lack of specifics, I offer it without comment.
USDA Builds on Actions to Protect Livestock and Public Health from H5N1 Avian Influenza
Press Release
WASHINGTON, October 30, 2024 – The U.S. Department of Agriculture’s Animal and Plant Health Inspection Service (APHIS) today announced the agency is planning to enhance testing and monitoring for H5N1, building on measures taken by USDA since the beginning of the avian influenza outbreak. In partnership with state veterinarians, USDA will implement a tiered strategy to collect milk samples to better assess where H5N1 is present, with the goal to better inform biosecurity and containment measures, as well as to inform state-led efforts to reduce risk to farm workers who may be in contact with animals infected with H5N1.
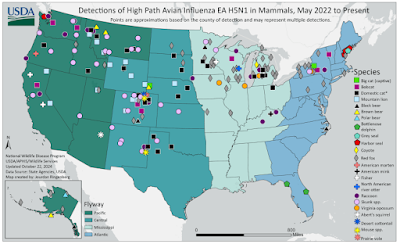
Since this disease was first detected in dairy cattle in March 2024, the USDA and state and federal partners have taken several steps to better understand the virus and work to eliminate it from dairy herds. In May 2024, USDA implemented a Federal Order to require the testing of cattle before interstate movement, which has helped to limit H5N1’s spread to new states; in the past 30 days, the number of states with known avian influenza detections in dairy herds has dropped from 14 to two. However, USDA believes that additional steps are needed to proactively support effective biosecurity measures, which are key for states and farmers to contain and eliminate H5N1 infections from their livestock.
USDA has precedent with successful bulk milk testing approaches, including the use of bulk milk testing to eradicate brucellosis from dairy herds. In addition, Colorado implemented statewide bulk milk testing after H5N1 was detected in dairy herds in two counties, and the most recent statewide testing has not detected any evidence of H5N1 in any herds in the state. In the coming weeks, USDA will work with regions and states that are ready to assist in expanding bulk milk testing.
USDA is working closely with state and private veterinary groups, which include practitioners who will play a vital role in carrying out this effort. USDA plans to first sample milk in bulk at the regional level, with additional testing at the farm level if necessary, until herds in an area are determined to be free of the virus. USDA will continue to work with state and private veterinarians on the final details of implementation, and will share guidance documents soon.
USDA continues to emphasize to farmers nationwide that biosecurity is the best weapon against the spread of H5N1, and farms should practice good biosecurity even if the virus has not been detected in their state or vicinity. Data collected over the past seven months has shown that H5N1 can be transmitted on equipment, people, or other items that move from farm to farm, including between dairies and poultry facilities. USDA’s Federal Order, announced in April 2024, to require testing before cattle movement between states has helped limit the spread of H5N1 to only 14 states, but local and state efforts to enhance biosecurity measures are also important. USDA strongly encourages herd owners to participate in available producer support programs, which help to cover the cost such as biosecurity programming, PPE for employees, and veterinary care.
In addition, USDA continues to support the rapid development and timely approval of an H5N1 vaccine for dairy cows, in addition to other species. Two vaccine candidates for use in dairy cows are currently undergoing field trials.
USDA has consistently operated on a science-based, step-by-step approach informed by what it learns about this virus through its everyday work, research, and monitoring efforts, and this marks the next step in the escalation of the agency’s response.
Today, USDA and the Oregon Department of Agriculture also announced the first detection of H5N1 in swine in the United States, which was detected in a non-commercial farm operation in Oregon. More information that announcement can be found here.
USDA scientists have worked closely with colleagues at the Centers for Disease Control and Prevention (CDC) and across the country and have not found any recent changes to the virus that increase the risk of transmission from animals to humans or between people. While cases among humans in direct contact with infected animals do continue to occur, partners at the CDC believe that the current risk to the public remains low.
As USDA takes additional steps to protect the health of livestock, the Department will continue to work closely with its federal partners at CDC to protect the health of people and FDA to protect the safety of the food supply. These collective, collaborative efforts have helped protect farmworkers and farmers, the health and welfare of livestock animals, and reaffirmed the safety of the nation’s food supply. The U.S. government remains committed to addressing this situation with urgency.
To learn more about USDA’s response to HPAI in dairy cattle, visit www.aphis.usda.gov/livestock-poultry-disease/avian/avian-influenza/hpai-detections/livestock.