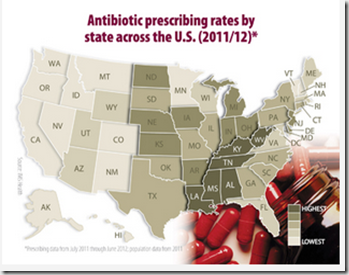
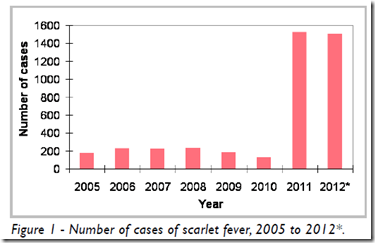
Credit CDC PHIL
# 8856
Because it is such an international city, and boasts one of the most diligent (and open) public health agencies in the world (Centre For Health Protection), Hong Kong has become a terrific barometer for the growth of multiple drug resistant infections from around the world.
One of the toughest bacteria that hospitals must deal with is called multidrug-resistant (MDR) Acinetobacter baumannii, which in recent years has made headlines as the cause of difficult to treat wound infections among our troops serving in the Middle East.
Acinetobacter (of which there are many varieties, but A. baumannii is most often linked to human infection ) are ubiquitous in nature, and can be found in soil, water, animals and humans. A very hardy species, they can survive for extended period of time on inanimate surfaces, making them difficult to control in a health care setting (see AJIC report Hospital cleaning protocol ineffective against A. baumannii)..
And like with MRSA, many people can be colonized, but not show signs of infection. Often very serious infections develop among those who are very ill, wounded, or immunocompromised.
Today Hong Kong’s CHP has published two reports on hospital clusters of MDR Acinetobacter infection.
Cluster of Multi-drug Resistant Acinetobacter cases in Queen Elizabeth Hospital
The following is issued on behalf of the Hospital Authority:
The spokesperson for Queen Elizabeth Hospital (QEH) made the following announcement today (July 22):
Five male patients (aged 35 to 80) of a Ventilator Ward have been confirmed as having Multi-drug Resistant Acinetobacter (MDRA) since July 14. Two of them are infected cases and are still hospitalised at QEH. The remaining three patients were confirmed to be MDRA carriers without clinical symptoms. Out of these cases, two are still hospitalised under medical surveillance and isolation at QEH. The remaining patient has been transferred to Hong Kong Buddhist Hospital. All of the five patients are in stable condition.Cluster of Multi-drug Resistant Acinetobacter cases in Caritas Medical Centre
The following is issued on behalf of the Hospital Authority:
The spokesperson of Caritas Medical Centre made the following announcement today (July 22):
Three patients (aged 37 to 88) of a male Medicine and Geriatrics Ward have been confirmed as having Multi-drug Resistant Acinetobacter (MDRA) since July 17. Two of them are infected cases. The patients are still hospitalised under medical surveillance and isolation. Two of them are in stable condition, while the other one is in serious condition.
Just yesterday, Hong Kong reported a Case of NDM-5 Carbapenemase-producing Enterobacteriaceae under CHP investigation in a 30-year-old woman with a urinary tract infection.
NDM-5 is a novel variant of the NDM-1 enzyme which first made headlines four years ago when The Lancet published a study (see NDM-1: A New Acronym To Memorize) by Walsh, Toleman, Livermore, et al. on the emergence and growing prevalence of the antibiotic resistant enzyme on the Indian sub-continent.
While still relatively rare – at least in the United States and Europe – this growing rogues gallery of new, multi-drug resistant organisms continues to gain traction around the world, threatening an early demise for much of our current antibiotic arsenal.
In early 2012 World Health Director-General Margaret Chan expressed a dire warning about our dwindling antibiotic arsenal (see Chan: World Faces A `Post-Antibiotic Era’) – a sentiment echoed a year later by CDC Director Thomas Frieden during the release of a major US report on the threat (see McKenna On CDC Antibiotic Resistance Report).
Dark, if not Inevitable conclusions, backed up by a long list of reports and studies showing the inexorable erosion the effectiveness of our current antibiotics to deal with rapidly evolving bacteria. Some of these reports I’ve covered in the past include:
EID Journal: Acquisition of Drug Resistant Genes Through International Travel
AAP/CDC: New Guidance On For Antibiotics For Children
The Lancet: Antibiotic Resistance - The Need For Global Solutions
UK CMO: Antimicrobial Resistance Poses `Catastrophic Threat’
MMWR Vital Signs: Carbapenem-Resistant Enterobacteriaceae (CRE)
For a more complete look at the complex issues of antibiotic resistance, and the dearth of new drugs on the horizon, I can think of no resource better than Maryn McKenna’s superb book (and recent winner of the 2013 June Roth Memorial Book Award, American Society of Journalists and Authors) Superbug: The Fatal Menace of MRSA.
And while I dabble in writing about the issues of antibiotic resistance, undoubtedly the best coverage can be found on Maryn’s Superbug blog.