# 6638
Two days ago Michael Osterholm and his group at CIDRAP released their 160-page Comprehensive Influenza Vaccine Initiative (CCIVI) report, that among other things, cited longstanding overstatement of the effectiveness of the seasonal flu vaccine as a barrier to creating new, and more efficient vaccine technology.
Up until about a year ago the CDC’s mantra has been for healthy adults under the age of 65, in years when the vaccine is a good match to circulating strains, effectiveness ranges from 70%-90%.
A statement that at times was interpreted as `up to 90% effective’ by officials and the media. A quick Google this morning found the following statement on a major company’s website (link) from 2006.
Get a seasonal flu shot every year. The Centers for Disease Control and Prevention (CDC) report that getting a seasonal flu shot the best way to prevent the seasonal flu. In fact it's up to 90% effective in preventing the seasonal flu and even if you catch the seasonal flu, the immunity provided by the vaccine can make your case milder.
Not only does this site overstate the effectiveness of the seasonal flu jab, it fails to mention the CDC’s disclaimer of `in healthy adults under the age of 65’. I can find plenty of instances of this 90% effectiveness meme being used, some as recently as August of this year (link)
A little more than a year ago the CDC updated their FAQ on Flu Vaccine effectiveness, and as part of a much longer detailed posting, lowered their estimate of the inactivated flu shot’s effectiveness to read:
. . . recent RCTs of inactivated influenza vaccine among adults under 65 years of age have estimated 50-70% vaccine efficacy during seasons in which the vaccines' influenza A components were well matched to circulating influenza A viruses.
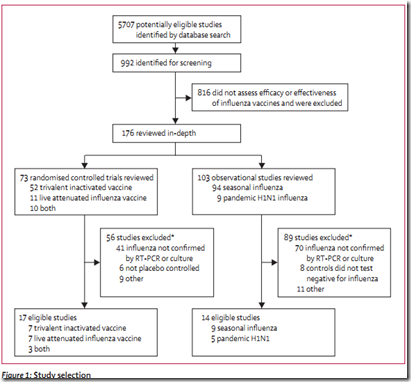
A number that pretty much matched CIDRAP’s finding (see A Comprehensive Flu Vaccine Effectiveness Meta-Analysis) which would be released a couple of weeks later. That analysis showed the trivalent inactivated vaccine (TIV) had a combined efficacy of 59% among healthy adults (aged 18–65 years).
While these numbers are much lower than we would would like to see, 50%-60% protection is far superior to no protection at all.
Which is why I continue to get, and support getting, the seasonal flu vaccine.
All of which serves as prelude to some extended comments released yesterday by Dr. Peter Sandman on the CCIVI report and public health’s long-standing inclination to overstate the effectiveness of the flu vaccine.
For those unfamiliar with Dr. Sandman, he is a world renown expert on crisis communications, who along with his wife and colleague Dr. Jody Lanard, provide consulting services to individuals, organizations, and companies – often during their worst public relations nightmares.
Together they also produce a wealth of invaluable risk management advice on their website, which quite frankly should be second home for anyone involved in public relations or risk communications.

In the interests of full disclosure Dr. Sandman served on the CCIVI Expert Advisory Group and has worked with CIDRAP in various capacities in the past, points that he makes abundantly clear in his preface.
What follows are excerpts from a lengthy email he sent to Lisa Schnirring of CIDRAP NEWS, in advance of the report’s release, for use in her news articles.
There is so much good content here, I find it difficult to pick and choose excerpts. As you’ll see, from the title onward, Dr. Sandman does not mince words - so please - follow the link to read it in its entirety.
We’d Be Likelier to Develop a Better Flu Vaccine If Public Health Officials Didn’t Keep Misleading Everyone about the Flu Vaccine We Have
by Peter M. Sandman
(an October 14, 2012 email to Lisa Schnirring of CIDRAP News)
On October 15, 2012, CCIVI released its report, entitled “The Compelling Need for Game-Changing Influenza Vaccines.” The report argued that the current flu vaccine is sorely inadequate; that a key barrier to developing a better vaccine is the widespread judgment that the current one is fine; and that the main reason the vaccine’s effectiveness is so consistently overestimated is that public health officials keep saying it is better than it is.
<SNIP>
Chapter 7 does a fine job of documenting how public health – especially ACIP – overestimates and overstates the efficacy of the flu vaccine. There are really three criticisms here:
- ACIP recommendations for ever-wider flu vaccination have been grounded in claims, assumptions, and judgments that the vaccine was more effective than it actually is.
- Early on that was because good data weren’t available, but long after there were ever-better data showing that the flu vaccine wasn’t very effective, ACIP continued to speak and act as if it were – ignoring some studies, misinterpreting others, leaning too heavily on studies with big methodological flaws, relying on plausibility and expert judgment while claiming to be relying on sound science, etc.
- In their zeal to encourage vaccination, ACIP, CDC, and the rest of the public health leadership kept telling the public (often via state and local public health officials and people’s personal doctors) that the flu vaccine worked better than it works.
<SNIP>
2. How do you think the report will be received? (Some of Chapter 7 sure reads like a GAO report. Lots of investigation work went into the analysis of ACIP’s recommendations.) What areas might see some early impact from the findings?
The central claim in the report is of course its claim that the flu vaccine is a lot less effective than most vaccines and a better one is badly needed.
Many in public health will find that claim difficult to embrace. But however reluctantly, I think they will embrace it. The Lancet I.D. study paved the way; in anticipation of that study’s publication, CDC stopped claiming 70–90% effectiveness in healthy adults under 65 and retreated to the much more supportable 50–70% estimate.
Now, sadly, CDC and many lower-level public health officials often provide no flu vaccine effectiveness estimate at all in their public communications, having learned that 70–90% is scientifically unsound but fearful that the more accurate 50–70% might undermine public acceptance. This is a small example of officials not trusting the public, which is a very large risk communication problem in public health. (See “Trust the Public with More of the Truth: What I Learned in 40 Years in Risk Communication.”)
(Continue . . .)
The CCIVI report and Dr. Sandman’s comments will undoubtedly discomfit many in the public health field, even if they privately accept their findings.
There is, after all, legitimate concern that anti-vaccine activists will use this report as fodder for their propaganda machine.
But in reality, public health faces an even bigger challenge.
As I wrote earlier this year in in Science At The Crossroads, the public’s faith in science and technology is eroding. And during a public health emergency, that could prove disastrous.
One only has to look at the deep divisions over climate change, evolution, vaccine safety, nuclear power, and genetically modified food crops to realize just how wide this rift between the public and scientists has become.
Recent revelations regarding deceit and fraud in scientific research (see PNAS study Misconduct accounts for the majority of retracted scientific publications) have only served to intensify this mistrust.
Rekindling the public’s trust is paramount, and the first step in that direction is trusting the public with the truth (or at least, our best estimation of the truth at the time).
If the vaccine is only 60% effective, we need to embrace that number and promote it the same way we do seatbelts.
Seatbelts don’t guarantee you’ll walk away from a wreck, but they sure improve your odds.
Most people understand that, and buckle up.
I honestly believe that those who are inclined to get a flu shot will accept those limitations, while those who are vehemently against vaccines . . . well, they weren’t going to be persuaded by VE numbers, no matter how high they were.
I can’t help but remember what a terrific job the CDC’s Admiral Anne Schuchat - Director of the National Center For immunization and Respiratory Diseases - did during the summer and fall of 2009 briefing the press and the public day after day on the emerging H1N1 pandemic.
Her candor, ability to work `off script’ and willingness to concede the things they did not know about the virus were equal parts effective, comforting, and refreshing - and in my mind, anyway – constituted the CDC’s finest hour during that crisis.
I believe this type of straight talk should be the model for all public health messaging, even if inconvenient facts (like a VE rate of 60%) are less than comforting.
That it is only if you trust the public with the truth that you can win, and hold, their confidence.
Anything less just deepens the rift of public distrust and plays into the hands of the critics.









![Reassortant pig[6] Reassortant pig[6]](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEgppTv7U9dZhh_wVqRdXedk6ElNkgm4B-LSTvmtqAyWYERTknTtfF1FxNLm3GMOlYI6MohfGUmrDBDKSXbAQ22-pDR7LRpCGc9u7IuTcnNUTQVm2hSh0YiTbRFxY5zVhkKqLbG_Ng/?imgmax=800)