# 9812
On a day that has hardly lacked for avian flu news (see APHIS: HPAI H5N2 Confirmed In Arkansas Turkey Flock & WHO: Updated H5N1 Cumulative Case Count Table), perhaps the most eagerly awaited story has been the publication of an analysis of the H7N9 virus in China, authored by a veritable `Who’s Who’ of virology.
Since both Declan Butler and Debra Mackenzie have already published overviews, I’ll avoid re-inventing the wheel and post the link to the article followed by links to both of their reports.
Dissemination, divergence and establishment of H7N9 influenza viruses in China
Tommy Tsan-Yuk Lam, Boping Zhou, Jia Wang, Yujuan Chai, Yongyi Shen, Xinchun Chen, Chi Ma, Wenshan Hong, Yin Chen, Yanjun Zhang, Lian Duan, Peiwen Chen, Junfei Jiang, Yu Zhang, Lifeng Li, Leo Lit Man Poon, Richard J. Webby, David K. Smith, Gabriel M. Leung, Joseph S. M. Peiris, Edward C. Holmes, Yi Guan & Huachen Zhu
Since 2013 the occurrence of human infections by a novel avian H7N9 influenza virus in China has demonstrated the continuing threat posed by zoonotic pathogens1, 2. Although the first outbreak wave that was centred on eastern China was seemingly averted, human infections recurred in October 2013 (refs 3, 4, 5, 6, 7). It is unclear how the H7N9 virus re-emerged and how it will develop further; potentially it may become a long-term threat to public health. Here we show that H7N9 viruses have spread from eastern to southern China and become persistent in chickens, which has led to the establishment of multiple regionally distinct lineages with different reassortant genotypes. Repeated introductions of viruses from Zhejiang to other provinces and the presence of H7N9 viruses at live poultry markets have fuelled the recurrence of human infections. This rapid expansion of the geographical distribution and genetic diversity of the H7N9 viruses poses a direct challenge to current disease control systems. Our results also suggest that H7N9 viruses have become enzootic in China and may spread beyond the region, following the pattern previously observed with H5N1 and H9N2 influenza viruses8, 9.
Declan Butler’s piece in Nature News:
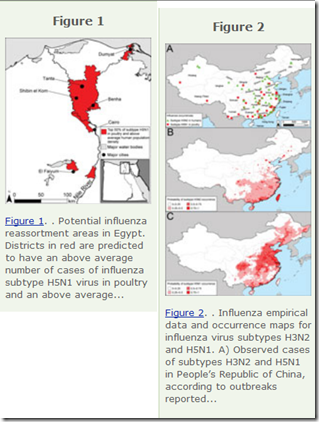
Flu genomes trace H7N9's evolution and spread in China
But surveillance of avian influenza viruses is patchy and slow.
11 March 2015
No one knows whether the H7N9 avian influenza that has infected more than 560 people in China and killed 204 might yet evolve to spread easily among people. But the largest-ever genomic survey of the virus in poultry now provides a more detailed picture of its evolution and and spread.
And from Debra McKenzie at New Scientist has this take:
Threatwatch: Bird flu's back and it's brought friends
- 18:00 11 March 2015 by Debora MacKenzie
- For similar stories, visit the Bird Flu Topic Guide
Threatwatch is your early warning system for global dangers, from nuclear peril to deadly viral outbreaks. Debora MacKenzie highlights the threats to civilisation – and suggests solutions
H5N1 bird flu burst out of China in 2003 and stormed across Eurasia and into Africa three years later. It's been there ever since and this week its victims are Egyptians. Now another strain of bird flu, H7N9, is spreading in China and the signs are that it might soon rampage across the continent just like H5N1.
The growing diversity of – and perceived threat from – the H7N9 virus isn’t new. We’ve been following its progress for several years, in studies such as:
PNAS: Evolution Of H9N2 And It’s Effect On The Genesis Of H7N9
Eurosurveillance: Genetic Tuning Of Avian H7N9 During Interspecies Transmission
EID Journal: H7N9 As A Work In Progress
And practically each step along the way, we’ve been warned:
Overall, due to the genetic tuning procedure, the potential pandemic risk posed by the novel avian influenza A(H7N9) viruses is greater than that of any other known avian influenza viruses.
While today’s study in Nature supports this notion, coming up from behind are a plethora of HPAI H5 viruses (H5N1, H5N2, H5N3,H5N6, H5N8), which have made a dramatic showing the past few months, particularly in Egypt, where we are seeing the worst human outbreak of H5N1 since that virus emerged more than a decade ago.
Add in the continually reassorting HPAI H5 variants spreading rapidly around the globe, and 2 weeks ago we saw a statement from WHO: H5 Currently The Most Obvious Avian Flu Threat.
While we can debate whether H7 or H5 has the greatest chance of sparking a pandemic, the bottom line is that with this large of a novel flu field in place, the risks of having one of them make that leap are probably better than they have been in years.