#16,723
On Thursday night the CDC announced the first
U.S. Case of Human Avian Influenza A(H5) in a person who was involved in the culling (
depopulating) of poultry with presumptive H5N1 bird flu. The patient - who was treated with oseltamivir - reported fatigue for several days, but has since recovered.
It isn't entirely clear whether this person was actually infected, or whether this person's positive nasal swab picked up surface contamination, but for now this patient meets the CDC criteria for a presumptive case.
The Eurasian H5N1 virus - which is currently spreading in wild birds and in poultry across the United States (and Europe) - is a far cry from Asian H5N1 virus that caused nearly 900 human infections - and hundreds of deaths - in Asia and the Middle East during the first two decades of the 21st century.
But over the past couple of years we've seen subtle signs that Eurasian H5Nx (e.g. H5N1, H5N8, H5N6, etc.) viruses may be slowly evolving towards becoming a greater human threat.
In early 2021 Russia announced 7 infections among poultry workers, which convinced the CDC To Add Zoonotic Avian A/H5N8 To Their IRAT List last May. Last December the ECDC/EFSA Raised the Zoonotic Risk Potential Of Avian H5Nx, following the detection of a human infection in the UK.
Over the past 18 months we've also seen an increasing number of reports of mammalian infections with the Eurasian H5Nx virus, resulting in severe illness and neurological manifestations (see here, here, and here).
While the CDC has repeatedly stated that the H5N1 Bird Flu Poses Low Risk to the Public they have also warned that ". . . sporadic human infections with current H5N1 bird flu viruses would not be surprising, especially among people with exposures who may not be taking recommended precautions (like wearing personal protective equipment, for example).
Now that a presumptive human case has been identified (and reportedly, 10 others have been offered antivirals and are being monitored), the CDC has released a HAN Health Advisory for clinicians and public health officials on identifying and investigating additional suspected cases.
Because of its length, I've only include some excerpts from this HAN advisory. Follow the link to read it in its entirety. I'll have a brief postscript when you return.
Highly Pathogenic Avian Influenza A(H5N1) Virus: Recommendations for Human Health Investigations and Response
Distributed via the CDC Health Alert Network
Friday, April 29, 2022, 8:00 PM ET
CDCHAN-00464
Summary
A person has tested positive for avian influenza A(H5) virus (H5 bird flu) in the U.S., as confirmed by the Centers for Disease Control and Prevention (CDC) and reported by the Colorado Department of Public Health and Environment on April 28, 2022. This case occurred in a person who had direct exposure to poultry and who was involved in the culling (depopulating) of poultry with presumptive H5N1 bird flu.
Starting in January, the U.S. Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service (APHIS) detected highly pathogenic avian influenza (HPAI) A(H5N1) virus in wild birds in the United States followed by multiple detections in U.S. commercial poultry and backyard bird flocks [1,2]. Detection of A(H5) virus in one person who was involved in culling of poultry does not change the human health risk assessment, which remains low for the general public. People with work or recreational exposures to infected birds are at greater risk of infection and should follow recommended precautions.
The purpose of this HAN Health Advisory is to notify public health workers, clinicians, and the public of the potential for human infection with this virus and to describe the CDC’s recommendations for patient investigation and testing, infection control including the use of personal protective equipment, and antiviral treatment and prophylaxis.
Background
(SNIP)
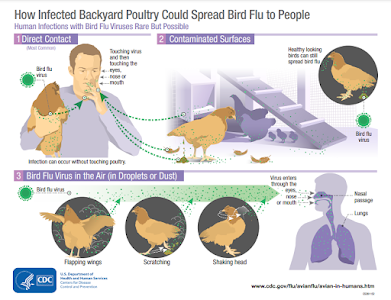
Influenza A viruses infect the respiratory and gastrointestinal tracts of birds causing birds to shed the virus in their saliva, mucous, and feces. Human infections with avian influenza A viruses can happen when enough virus gets into a person’s eyes, nose, or mouth or is inhaled. People with close or prolonged unprotected contact with infected birds or contaminated environments are at greater risk of infection. Illnesses in humans from avian influenza A virus infections have ranged from mild (e.g., eye infection, upper respiratory symptoms) to severe illness (e.g., pneumonia) resulting in death. The spread of avian influenza A viruses from one infected person to another has been reported in other countries, but is very rare, and when it has happened, it has not led to sustained spread among people.
At this time, CDC considers the human health risk to the U.S. public from these newly identified HPAI A(H5N1) viruses to be low; however, people with close or prolonged, unprotected contact with infected birds or contaminated environments are at greater risk of infection. While there is little information about the spectrum of illness that could result from human infections with current H5N1 bird flu viruses, currently, CDC considers this virus as having the potential to cause severe disease in humans and recommends the following:
Recommendations for Clinicians
Clinicians should consider the possibility of HPAI A(H5N1) virus infection in persons showing signs or symptoms of respiratory illness who have relevant exposure history. This includes persons who have had contact with potentially infected birds (e.g., handling, slaughtering, defeathering, butchering, culling, preparation for consumption); direct contact with water or surfaces contaminated with feces or parts (carcasses, internal organs, etc.) of potentially infected birds; and persons who have had prolonged exposure to potentially infected birds in a confined space. Clinicians should contact the state public health department to arrange testing for influenza A(H5N1) virus, collect respiratory specimens using personal protective equipment (PPE), consider starting empiric antiviral treatment (see below), and encourage the patient to isolate at home away from their household members and not go to work or school until it is determined they do not have avian influenza A virus infection. Testing for other potential causes of acute respiratory illness should also be considered depending upon the local epidemiology of circulating respiratory viruses, including SARS-CoV-2.
Recommendations for State Health Departments
State health departments should investigate potential human cases of HPAI A(H5N1) virus infection as described below and should notify CDC within 24 hours of identifying a case under investigation. Rapid detection and characterization of novel influenza A viruses in humans remain critical components of national efforts to prevent further cases, to allow for evaluation of clinical illness associated with them, and to assess the ability of these viruses to spread from human to human.
Recommendations for Surveillance and Testing
People exposed to HPAI A(H5N1)-infected birds (including people wearing recommended PPE) should be monitored for signs and symptoms of influenza beginning after their first exposure and for 10 days after their last exposure.
Patients who meet Epidemiologic criteria AND either Clinical OR Public Health Response criteria below should be tested for HPAI A(H5N1) virus infection by reverse-transcription polymerase chain reaction (RT-PCR) assay using H5-specific primers and probes at your state or local public health department.
(SNIP)
Recommendations for the Public
People should avoid unprotected exposure to sick or dead birds, bird feces, litter, or materials contaminated by birds with suspected or confirmed HPAI A(H5N1) virus infection. Personal protective equipment (PPE) includes a properly fitted unvented or indirectly vented safety goggles, disposable gloves, boots or boot covers, a NIOSH-approved respirator (e.g., N95), disposable fluid-resistant coveralls, and disposable head cover or hair cover. PPE should be worn when in direct or close contact (within about six feet) with sick or dead poultry, poultry feces, litter, or materials potentially contaminated with HPAI A(H5N1) virus.
People exposed to HPAI A(H5N1)-virus infected birds (including people wearing recommended PPE) should monitor for signs and symptoms of influenza beginning after their first exposure and for 10 days after their last exposure. Influenza antiviral prophylaxis may be considered to prevent infection, particularly in those who had unprotected exposure to HPAI A(H5N1)-virus infected birds (see below). Persons who develop respiratory illness after exposure to HPAI A(H5N1) virus infected birds should seek prompt medical evaluation for influenza testing and antiviral treatment by their clinician or public health department. Symptomatic persons should isolate away from household members and others except for seeking medical evaluation.
(Continue . . . )
When I began this blog more than 16 years ago, a far more dangerous ancestor of H5N1 was perceived as posing an imminent, and potentially devastating pandemic threat. Although H5N1 faltered - and eventually devolved into less dangerous variants - we have seen 2 unexpected pandemics emerge in the interim (H1N1 in 2009, and SARS-CoV-2 in 2020).
Today, there are arguably more credible pandemic threats on our radar than ever before.
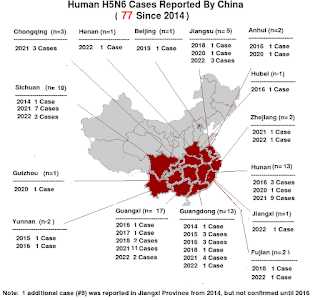
MERS-CoV, China's avian H5N6 virus, EA H1N1 `G4' swine flu, and the recently reported H3N8 virus in China all likely pose a greater public health threat than does H5N1, but the lesson of the past 20 years has been that we aren't very good at identifying the next pandemic threat.
The one thing we can say with reasonable certainty is that another pandemic will occur, and it may even arrive before our current Coronavirus crisis has ended.
So we must treat every species jump seriously.