Source Icelandic Met Office
UPDATED: See update at bottom.
# 9022
The on again, off again RED aviation alert for the Bárðarbunga volcano is on again after a 1.5 km long fissure eruption overnight, but right now it is impossible to know just how disruptive this event is likely to become. That said, this eruption appears to be much larger than the small fissure eruption on Friday.
For now, a long dyke of sub-surface magma stretches north from the volcano to Askja to the north, which is now on Yellow Alert.
A tweet from a team on site from the University of Iceland describes the event:
An Olympic-sized pool contains about 2500 cubic meters of water, so the estimated lava flow at this time would fill about 24 pools a minute, or 1440 pools an hour. Impressive, but at this time, this eruption appears to be smooth, non-explosive, and producing little or no ash.
The Icelandic Met Office – which warned yesterday that an eruption appeared more likely – describes the overnight event as:
31st August 2014 08:40 - Eruption in Holuhraun observed 05:15
Observation from scientists in the field (05:15): It appears that the eruptive fissure is longer than in the last eruption. It is extending north and south on the same fissure. The eruption is a very calm lava eruption and can hardly be seen on seismometers (almost no gosórói). Visual observation confirm it is calm, but continuous.
Several of the webcams monitoring the event I’ve mentioned in the past appear to be offline right now, but you can watch live (from a distance) on this webcam.
Seismic activity in and around Bárðarbunga continues, with a M5.1 reported within the past hour.

We should see an update from the
Stay tuned.
UPDATE: 1100 hrs EDT
Although weather conditions are hindering observations, the Scientific Advisory board has released the following update:
31st August 2014 12:07 - from the Scientific Advisory Board
Scientists from the Icelandic Met Office and the Institute of Earth Sciences and representatives of the Civil Protection in Iceland attend the meetings of the Scientific Advisory Board of the Icelandic Civil Protection.
Conclusions of the Scientific Advisory Board of the Icelandic Civil Protection:
- A lava eruption started in Holuhraun shortly after 04 AM, on the same volcanic fissure, which erupted earlier this week. The fissure is estimated to be 1,5 km long. It was detected on Míla´s web-camera at 05:51 AM. Fewer earthquakes seem to follow the event than in the previous eruption, but more lava is being extruded.
- At 07 AM the lava flow was around 1 km wide and 3 km long towards northeast. The thickness was estimated a few meters, the flow about 1000 m3 pr second.
- Approximately 500 earthquakes were detected in the area and smaller than before. The strongest earthquake, M3.8 was in the Bárðarbunga caldera. Poor weather conditions prevail in the area, which makes detection of smaller earthquakes difficult.
- GPS measurements show continued movements north of Dyngjujökull.
- Gas emissions rise to a few hundred meters above the fissure.
- Weather conditions make it difficult to follow the progression of the eruption, but scientists are in the area, using every opportunity to acquire information on gas and lava outflow.
- Weather conditions do not allow overflight at this time. The opportunity to fly over the area will be assessed later today.
From the Icelandic Met Office:
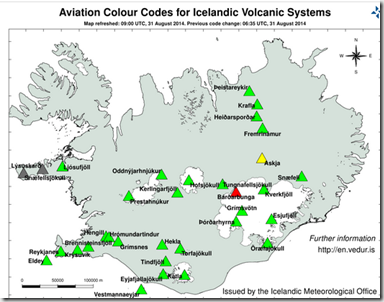
The Aviation Colour Code for Bárðarbunga is at ‘red' and the code for Askja at ‘yellow'.
I would note that the latest Aviation Color code map has just recently been changed back to Orange.